Leveraging 3D Bioprinting and Photon-Counting Computed Tomography to Enable Noninvasive Quantitative Tracking of Multifunctional Tissue Engineered Constructs
- PMID: 37709282
- PMCID: PMC10842604
- DOI: 10.1002/adhm.202302271
Leveraging 3D Bioprinting and Photon-Counting Computed Tomography to Enable Noninvasive Quantitative Tracking of Multifunctional Tissue Engineered Constructs
Abstract
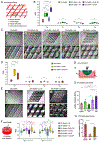
3D bioprinting is revolutionizing the fields of personalized and precision medicine by enabling the manufacturing of bioartificial implants that recapitulate the structural and functional characteristics of native tissues. However, the lack of quantitative and noninvasive techniques to longitudinally track the function of implants has hampered clinical applications of bioprinted scaffolds. In this study, multimaterial 3D bioprinting, engineered nanoparticles (NPs), and spectral photon-counting computed tomography (PCCT) technologies are integrated for the aim of developing a new precision medicine approach to custom-engineer scaffolds with traceability. Multiple CT-visible hydrogel-based bioinks, containing distinct molecular (iodine and gadolinium) and NP (iodine-loaded liposome, gold, methacrylated gold (AuMA), and Gd2 O3 ) contrast agents, are used to bioprint scaffolds with varying geometries at adequate fidelity levels. In vitro release studies, together with printing fidelity, mechanical, and biocompatibility tests identified AuMA and Gd2 O3 NPs as optimal reagents to track bioprinted constructs. Spectral PCCT imaging of scaffolds in vitro and subcutaneous implants in mice enabled noninvasive material discrimination and contrast agent quantification. Together, these results establish a novel theranostic platform with high precision, tunability, throughput, and reproducibility and open new prospects for a broad range of applications in the field of precision and personalized regenerative medicine.
Keywords: 3D bioprinting; bioinks; contrast agents; longitudinal quantitative imaging; nanoparticles; photon-counting computed tomography; tissue engineering scaffolds.
© 2023 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures




References
-
- Sharma K, Mujawar MA, and Kaushik A, State-of-Art Functional Biomaterials for Tissue Engineering. Frontiers in Materials, 2019. 6.
-
- Kohane DS and Langer R, Polymeric biomaterials in tissue engineering. Pediatr Res, 2008. 63(5): p. 487–91. - PubMed
-
- Khademhosseini A, et al., Chapter 27 - Embryonic stem cells as a cell source for tissue engineering, in Principles of Tissue Engineering (Fifth Edition), Lanza R, et al., Editors. 2020, Academic Press. p. 467–490.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

