Microbial metabolomic responses to changes in temperature and salinity along the western Antarctic Peninsula
- PMID: 37709939
- PMCID: PMC10579395
- DOI: 10.1038/s41396-023-01475-0
Microbial metabolomic responses to changes in temperature and salinity along the western Antarctic Peninsula
Abstract
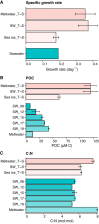
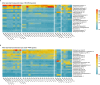
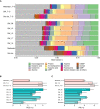
Seasonal cycles within the marginal ice zones in polar regions include large shifts in temperature and salinity that strongly influence microbial abundance and physiology. However, the combined effects of concurrent temperature and salinity change on microbial community structure and biochemical composition during transitions between seawater and sea ice are not well understood. Coastal marine communities along the western Antarctic Peninsula were sampled and surface seawater was incubated at combinations of temperature and salinity mimicking the formation (cold, salty) and melting (warm, fresh) of sea ice to evaluate how these factors may shape community composition and particulate metabolite pools during seasonal transitions. Bacterial and algal community structures were tightly coupled to each other and distinct across sea-ice, seawater, and sea-ice-meltwater field samples, with unique metabolite profiles in each habitat. During short-term (approximately 10-day) incubations of seawater microbial communities under different temperature and salinity conditions, community compositions changed minimally while metabolite pools shifted greatly, strongly accumulating compatible solutes like proline and glycine betaine under cold and salty conditions. Lower salinities reduced total metabolite concentrations in particulate matter, which may indicate a release of metabolites into the labile dissolved organic matter pool. Low salinity also increased acylcarnitine concentrations in particulate matter, suggesting a potential for fatty acid degradation and reduced nutritional value at the base of the food web during freshening. Our findings have consequences for food web dynamics, microbial interactions, and carbon cycling as polar regions undergo rapid climate change.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Meredith MP, King JC. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett. 2005;32:1–5.
-
- Arrigo KR, van Dijken G, Pabi S. Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett. 2008;35:1–6.
-
- Arrigo KR, Perovich DK, Pickart RS, Brown ZW, van Dijken GL, Lowry KE, et al. Phytoplankton blooms beneath the sea ice in the Chukchi sea. Deep Res Part II Top Stud Oceanogr. 2014;105:1–16.
-
- Cape MR, Vernet M, Pettit EC, Wellner J, Truffer M, Akie G, et al. Circumpolar deep water impacts glacial meltwater export and coastal biogeochemical cycling along the west Antarctic Peninsula. Front Mar Sci. 2019;6:1–23.
-
- Swart NC, Gille ST, Fyfe JC, Gillett NP. Recent Southern Ocean warming and freshening driven by greenhouse gas emissions and ozone depletion. Nat Geosci. 2018;11:836–41.

