Optimized glycemic control of type 2 diabetes with reinforcement learning: a proof-of-concept trial
- PMID: 37710000
- PMCID: PMC10579102
- DOI: 10.1038/s41591-023-02552-9
Optimized glycemic control of type 2 diabetes with reinforcement learning: a proof-of-concept trial
Abstract
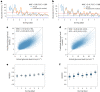
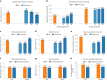
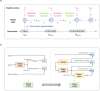
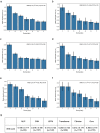
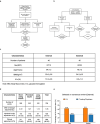
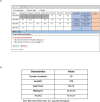
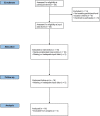
The personalized titration and optimization of insulin regimens for treatment of type 2 diabetes (T2D) are resource-demanding healthcare tasks. Here we propose a model-based reinforcement learning (RL) framework (called RL-DITR), which learns the optimal insulin regimen by analyzing glycemic state rewards through patient model interactions. When evaluated during the development phase for managing hospitalized patients with T2D, RL-DITR achieved superior insulin titration optimization (mean absolute error (MAE) of 1.10 ± 0.03 U) compared to other deep learning models and standard clinical methods. We performed a stepwise clinical validation of the artificial intelligence system from simulation to deployment, demonstrating better performance in glycemic control in inpatients compared to junior and intermediate-level physicians through quantitative (MAE of 1.18 ± 0.09 U) and qualitative metrics from a blinded review. Additionally, we conducted a single-arm, patient-blinded, proof-of-concept feasibility trial in 16 patients with T2D. The primary outcome was difference in mean daily capillary blood glucose during the trial, which decreased from 11.1 (±3.6) to 8.6 (±2.4) mmol L-1 (P < 0.01), meeting the pre-specified endpoint. No episodes of severe hypoglycemia or hyperglycemia with ketosis occurred. These preliminary results warrant further investigation in larger, more diverse clinical studies. ClinicalTrials.gov registration: NCT05409391 .
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


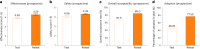



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

