Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial
- PMID: 37710005
- PMCID: PMC10579056
- DOI: 10.1038/s41591-023-02572-5
Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial
Erratum in
-
Publisher Correction: Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial.Nat Med. 2023 Oct;29(10):2665. doi: 10.1038/s41591-023-02624-w. Nat Med. 2023. PMID: 37814064 Free PMC article. No abstract available.
-
Author Correction: Axicabtagene ciloleucel as second-line therapy in large B cell lymphoma ineligible for autologous stem cell transplantation: a phase 2 trial.Nat Med. 2024 Jul;30(7):2089. doi: 10.1038/s41591-024-03053-z. Nat Med. 2024. PMID: 38745012 Free PMC article. No abstract available.
Abstract
Axicabtagene ciloleucel (axi-cel) demonstrated superior efficacy compared to standard of care as second-line therapy in patients with high-risk relapsed/refractory (R/R) large B cell lymphoma (LBCL) considered eligible for autologous stem cell transplantation (ASCT); however, in clinical practice, roughly half of patients with R/R LBCL are deemed unsuitable candidates for ASCT. The efficacy of axi-cel remains to be ascertained in transplant-ineligible patients. ALYCANTE, an open-label, phase 2 study, evaluated axi-cel as a second-line therapy in 62 patients with R/R LBCL who were considered ineligible for ASCT. The primary end point was investigator-assessed complete metabolic response at 3 months from the axi-cel infusion. Key secondary end points included progression-free survival, overall survival and safety. The study met its primary end point with a complete metabolic response of 71.0% (95% confidence interval, 58.1-81.8%) at 3 months. With a median follow-up of 12.0 months (range, 2.1-17.9), median progression-free survival was 11.8 months (95% confidence interval, 8.4-not reached) and overall survival was not reached. There was no unexpected toxicity. Grade 3-4 cytokine release syndrome and neurologic events occurred in 8.1% and 14.5% of patients, respectively. These results support axi-cel as second-line therapy in patients with R/R LBCL ineligible for ASCT. ClinicalTrials.gov Identifier: NCT04531046 .
© 2023. The Author(s).
Conflict of interest statement
R.H. has received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda and Roche; and is a member on an entity’s Board of Directors or advisory committees of Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte and Miltenyi. E.B. has received honoraria from Kite/Gilead, Bristol-Myers Squibb, Novartis, Pfizer, Incyte, ADC Therapeutics, Roche and Takeda; travel reimbursement from Kite/Gilead, Bristol-Myers Squibb, Novartis and Pfizer; and research funding from Amgen and Bristol-Myers Squibb. G.C. has received consulting fees from Roche, AbbVie, Bristol-Myers Squibb, MedXCell, Mabqi and Onward Therapeutics; honoraria from Jansen, Gilead, Novartis, Roche, Bristol-Myers Squibb and Incyte; and travel and accommodation expenses from Gilead, Roche and Jansen. F.X.G. has received consulting fees from Gilead, Bristol-Myers Squibb, Miltenyi and Novartis; and travel and accommodation expenses from Gilead and Novartis. F.M. has received consulting fees from Roche, Gilead, Novartis, Bristol-Myers Squibb, Genmab and AbbVie; and honoraria for advisory boards from Roche, Gilead and Miltenyi. L.O. has received consulting fees from Roche; honoraria from Bristol-Myers Squibb, Kite/Gilead and Incyte; and travel and accommodation expenses from Roche and AstraZeneca. T.G. has received consulting fees from Takeda and Kite/Gilead; honoraria from Kite/Gilead; and travel and accommodation expenses from Roche, Takeda and Kite/Gilead. P.F. has received consulting fees from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen; honoraria from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen; and travel and accommodation expenses from Gilead, AstraZeneca, BeiGene, AbbVie and Janssen. R.D. has received honoraria from Novartis and Takeda; research funding from Ligue contre le Cancer, Arthur Sachs, Monahan Foundation, Servier Foundation, Philippe Foundation and DCP AP-HP; and non-financial support from Kite/Gilead. C.T. has received institutional research funding from Kite/Gilead and Roche; honoraria for advisory boards from Roche, Novartis, AstraZeneca, BeiGene, AbbVie, Takeda, Roche, Novartis, Kite/Gilead, Bristol-Myers Squibb; and travel and accommodation expenses from Roche, Novartis, AbbVie, Takeda, Roche, Kite/Gilead and Bristol-Myers Squibb. F.J. has received honoraria from Roche, Gilead, Janssen and Bristol-Myers Squibb; honoraria for advisory boards from Roche; and travel and accommodation expenses from Roche and Gilead. S.C. has received consulting fees from Atara, Novartis, Kite/Gilead, Pierre Fabre, Takeda, AbbVie and AstraZeneca; honoraria for advisory boards from Kite/Gilead, Novartis, AbbVie, Takeda and Viatris; institutional funding from Janssen; and travel and accommodation expenses from Novartis, AbbVie and Pierre Fabre. O.C. has received honoraria from Roche, Takeda, Bristol-Myers Squibb, Merck, Kite/Gilead, AbbVie and ADC Therapeutics; and research funding from Roche, Takeda and Kite/Gilead. G.B. has received honoraria for advisory boards from Novartis, Kite/Gilead, Bristol-Myers Squibb and Incyte; and travel and accommodation expenses from Novartis, Kite/Gilead, Bristol-Myers Squibb and Incyte. M.C. has received institutional research funding from AP-HP, INSERM, INCA, Fondation ARC pour la Recherche sur le Cancer and CALYM; honoraria from Amgen and CSL Behring; research funding from Innate Pharma and Servier; and travel and accommodation expenses from Pfizer, Grifols, CSL Behring and Gilead. F.L.G. has received honoraria from Rennes University Hospital. C.M. has received research funding from Kite Pharmaceuticals. C.P. is a LYSARC employee. E.I. has received honoraria from Janssen-Cilag and Pfizer. C.L. has received research funding from Ligue contre le Cancer and Labex Toucan; and travel and accommodation expenses from Roche and Janssen. All other authors declare no competing interests.
Figures
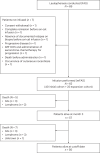





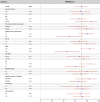
References
-
- Pfreundschuh M, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

