Crystal ribcage: a platform for probing real-time lung function at cellular resolution
- PMID: 37710017
- PMCID: PMC10860663
- DOI: 10.1038/s41592-023-02004-9
Crystal ribcage: a platform for probing real-time lung function at cellular resolution
Abstract
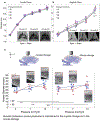
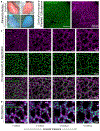
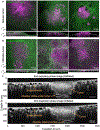
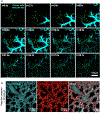
Understanding the dynamic pathogenesis and treatment response in pulmonary diseases requires probing the lung at cellular resolution in real time. Despite advances in intravital imaging, optical imaging of the lung during active respiration and circulation has remained challenging. Here, we introduce the crystal ribcage: a transparent ribcage that allows multiscale optical imaging of the functioning lung from whole-organ to single-cell level. It enables the modulation of lung biophysics and immunity through intravascular, intrapulmonary, intraparenchymal and optogenetic interventions, and it preserves the three-dimensional architecture, air-liquid interface, cellular diversity and respiratory-circulatory functions of the lung. Utilizing these capabilities on murine models of pulmonary pathologies we probed remodeling of respiratory-circulatory functions at the single-alveolus and capillary levels during disease progression. The crystal ribcage and its broad applications presented here will facilitate further studies of nearly any pulmonary disease as well as lead to the identification of new targets for treatment strategies.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Figures








References
-
- Entenberg D et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat Methods 15, 73–80 (2018). https://doi.org:10.1038/nmeth.4511 - DOI - PMC - PubMed
-
- Headley MB et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531, 513–517 (2016). https://doi.org:10.1038/nature16985 - DOI - PMC - PubMed
-
- Looney MR et al. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods 8, 91–96 (2011). https://doi.org:10.1038/nmeth.1543 - DOI - PMC - PubMed
-
- Ueki H, Wang IH, Zhao D, Gunzer M & Kawaoka Y Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS. Nat Protoc 15, 1041–1065 (2020). https://doi.org:10.1038/s41596-019-0275-y - DOI - PMC - PubMed
-
- Westphalen K et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506, 503–506 (2014). https://doi.org:10.1038/nature12902 - DOI - PMC - PubMed
Method-only references
-
- Muzumdar MD, Tasic B, Miyamichi K, Li L & Luo L A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007). https://doi.org:10.1002/dvg.20335 - DOI - PubMed
-
- Passegue E, Wagner EF & Weissman IL JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 119, 431–443 (2004). https://doi.org:10.1016/j.cell.2004.10.010 - DOI - PubMed
-
- Barker KA et al. Lung-resident memory B cells protect against bacterial pneumonia. J Clin Invest 131 (2021). https://doi.org:10.1172/JCI141810 - DOI - PMC - PubMed
-
- Kitzerow O, Zucker IH, Lisco SJ & Wang HJ Timeline of Multi-Organ Plasma Extravasation After Bleomycin-Induced Acute Lung Injury. Front Physiol 13, 777072 (2022). https://doi.org:10.3389/fphys.2022.777072 - DOI - PMC - PubMed
-
- Mammoto A et al. Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat Commun 4, 1759 (2013). https://doi.org:10.1038/ncomms2774 - DOI - PubMed
MeSH terms
Grants and funding
- R35 HL135756/HL/NHLBI NIH HHS/United States
- R21 EB031332/EB/NIBIB NIH HHS/United States
- T32 EB006359/EB/NIBIB NIH HHS/United States
- R01 HL171499/HL/NHLBI NIH HHS/United States
- R01 HL158732/HL/NHLBI NIH HHS/United States
- R01 AI162850/AI/NIAID NIH HHS/United States
- F30 HL168952/HL/NHLBI NIH HHS/United States
- S10 OD024993/OD/NIH HHS/United States
- R01 AI115053/AI/NIAID NIH HHS/United States
- T32 HL007035/HL/NHLBI NIH HHS/United States
- DP2 HL168562/HL/NHLBI NIH HHS/United States
- R01 HL158733/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources

