NO-ferroheme is a signaling entity in the vasculature
- PMID: 37710073
- PMCID: PMC10522487
- DOI: 10.1038/s41589-023-01411-5
NO-ferroheme is a signaling entity in the vasculature
Abstract
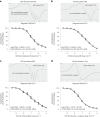
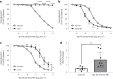
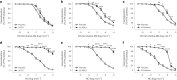
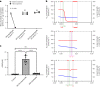
Despite wide appreciation of the biological role of nitric oxide (NO) synthase (NOS) signaling, questions remain about the chemical nature of NOS-derived bioactivity. Here we show that NO-like bioactivity can be efficiently transduced by mobile NO-ferroheme species, which can transfer between proteins, partition into a hydrophobic phase and directly activate the sGC-cGMP-PKG pathway without intermediacy of free NO. The NO-ferroheme species (with or without a protein carrier) efficiently relax isolated blood vessels and induce hypotension in rodents, which is greatly potentiated after the blockade of NOS activity. While free NO-induced relaxations are abolished by an NO scavenger and in the presence of red blood cells or blood plasma, a model compound, NO-ferroheme-myoglobin preserves its vasoactivity suggesting the physiological relevance of NO-ferroheme species. We conclude that NO-ferroheme behaves as a signaling entity in the vasculature.
© 2023. The Author(s).
Conflict of interest statement
A.L.K. was an employee of Freiberg Instruments GmbH and is the inventor of a patent related to diagnostic and therapeutic applications of NO-ferroheme (European patent number: 17186234.5). The other authors declare no competing interests.
Figures


References
-
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

