Sex-dependent neuronal effects of α-synuclein reveal that GABAergic transmission is neuroprotective of sleep-controlling neurons
- PMID: 37710341
- PMCID: PMC10500827
- DOI: 10.1186/s13578-023-01105-4
Sex-dependent neuronal effects of α-synuclein reveal that GABAergic transmission is neuroprotective of sleep-controlling neurons
Abstract
Background: Sleep disorders (SDs) are a symptom of the prodromal phase of neurodegenerative disorders that are mechanistically linked to the protein α-synuclein (α-syn) including Parkinson's disease (PD). SDs during the prodromal phase could result from neurodegeneration induced in state-controlling neurons by accumulation of α-syn predominant early in the disease, and consistent with this, we reported the monomeric form of α-syn (monomeric α-syn; α-synM) caused cell death in the laterodorsal tegmental nucleus (LDT), which controls arousal as well as the sleep and wakefulness state. However, we only examined the male LDT, and since sex is considered a risk factor for the development of α-syn-related diseases including prodromal SDs, the possibility exists of sex-based differences in α-synM effects. Accordingly, we examined the hypothesis that α-synM exerts differential effects on membrane excitability, intracellular calcium, and cell viability in the LDT of females compared to males.
Methods: Patch clamp electrophysiology, bulk load calcium imaging, and cell death histochemistry were used in LDT brain slices to monitor responses to α-synM and effects of GABA receptor acting agents.
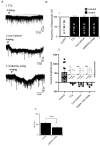
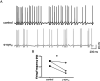
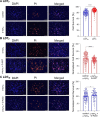
Results: Consistent with our hypothesis, we found differing effects of α-synM on female LDT neurons when compared to male. In females, α-synM induced a decrease in membrane excitability and heightened reductions in intracellular calcium, which were reliant on functional inhibitory acid transmission, as well as decreased the amplitude and frequency of spontaneous excitatory postsynaptic currents (sEPSCs) with a concurrent reduction in action potential firing rate. Cell viability studies showed higher α-synM-mediated neurodegeneration in males compared to females that depended on inhibitory amino acid transmission. Further, presence of GABA receptor agonists was associated with reduced cell death in males.
Conclusions: When taken together, we conclude that α-synM induces a sex-dependent effect on LDT neurons involving a GABA receptor-mediated mechanism that is neuroprotective. Understanding the potential sex differences in neurodegenerative processes, especially those occurring early in the disease, could enable implementation of sex-based strategies to identify prodromal PD cases, and promote efforts to illuminate new directions for tailored treatment and management of PD.
Keywords: Cholinergic; Laterodorsal Tegmentum; Sleep disorders; α -synucleinopathies, neurodegenerative disease.
© 2023. Society of Chinese Bioscientists in America (SCBA).
Conflict of interest statement
All authors disclose that they have no relevant financial or non-financial interests to disclose.
Figures


References
-
- de Lau LML, Giesbergen PCLM, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study, Neurology. 63 (2004) 1240–4. 10.1212/01.WNL.0000140706.52798.BE. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

