Identifying lupus Patient Subsets Through Immune Cell Deconvolution of Gene Expression Data in Two Atacicept Phase II Studies
- PMID: 37710418
- PMCID: PMC10570667
- DOI: 10.1002/acr2.11594
Identifying lupus Patient Subsets Through Immune Cell Deconvolution of Gene Expression Data in Two Atacicept Phase II Studies
Abstract
Objective: To use cell-based gene signatures to identify patients with systemic lupus erythematous (SLE) in the phase II/III APRIL-SLE and phase IIb ADDRESS II trials most likely to respond to atacicept.
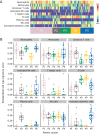
Methods: A published immune cell deconvolution algorithm based on Affymetrix gene array data was applied to whole blood gene expression from patients entering APRIL-SLE. Five distinct patient clusters were identified. Patient characteristics, biomarkers, and clinical response to atacicept were assessed per cluster. A modified immune cell deconvolution algorithm was developed based on RNA sequencing data and applied to ADDRESS II data to identify similar patient clusters and their responses.
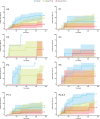
Results: Patients in APRIL-SLE (N = 105) were segregated into the following five clusters (P1-5) characterized by dominant cell subset signatures: high neutrophils, T helper cells and natural killer (NK) cells (P1), high plasma cells and activated NK cells (P2), high B cells and neutrophils (P3), high B cells and low neutrophils (P4), or high activated dendritic cells, activated NK cells, and neutrophils (P5). Placebo- and atacicept-treated patients in clusters P2,4,5 had markedly higher British Isles Lupus Assessment Group (BILAG) A/B flare rates than those in clusters P1,3, with a greater treatment effect of atacicept on lowering flares in clusters P2,4,5. In ADDRESS II, placebo-treated patients from P2,4,5 were less likely to be SLE Responder Index (SRI)-4, SRI-6, and BILAG-Based Combined Lupus Assessment responders than those in P1,3; the response proportions again suggested lower placebo effect and a greater treatment differential for atacicept in P2,4,5.
Conclusion: This exploratory analysis indicates larger differences between placebo- and atacicept-treated patients with SLE in a molecularly defined patient subset.
© 2023 EMD Serono, Inc and The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
Similar articles
-
Post Hoc Analysis of the Phase II/III APRIL-SLE Study: Association Between Response to Atacicept and Serum Biomarkers Including BLyS and APRIL.Arthritis Rheumatol. 2017 Jan;69(1):122-130. doi: 10.1002/art.39809. Epub 2016 Dec 2. Arthritis Rheumatol. 2017. PMID: 27390168 Free PMC article. Clinical Trial.
-
Safety and clinical activity of atacicept in the long-term extension of the phase 2b ADDRESS II study in systemic lupus erythematosus.Rheumatology (Oxford). 2021 Nov 3;60(11):5379-5389. doi: 10.1093/rheumatology/keab115. Rheumatology (Oxford). 2021. PMID: 33547784 Clinical Trial.
-
Efficacy and Safety of Atacicept in Patients With Systemic Lupus Erythematosus: Results of a Twenty-Four-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm, Phase IIb Study.Arthritis Rheumatol. 2018 Feb;70(2):266-276. doi: 10.1002/art.40360. Arthritis Rheumatol. 2018. PMID: 29073347 Free PMC article. Clinical Trial.
-
Belimumab: review of use in systemic lupus erythematosus.Clin Ther. 2012 May;34(5):1006-22. doi: 10.1016/j.clinthera.2012.02.028. Epub 2012 Mar 30. Clin Ther. 2012. PMID: 22464040 Review.
-
Profile of atacicept and its potential in the treatment of systemic lupus erythematosus.Drug Des Devel Ther. 2015 Mar 5;9:1331-9. doi: 10.2147/DDDT.S71276. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25834391 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

