Mechanism of a Dually Catalyzed Enantioselective Oxa-Pictet-Spengler Reaction and the Development of a Stereodivergent Variant
- PMID: 37711191
- PMCID: PMC10501388
- DOI: 10.1021/acscatal.2c05484
Mechanism of a Dually Catalyzed Enantioselective Oxa-Pictet-Spengler Reaction and the Development of a Stereodivergent Variant
Abstract
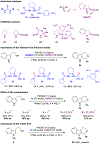
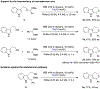
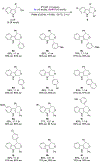
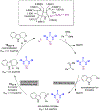
Enantioselective oxa-Pictet-Spengler reactions of tryptophol with aldehydes proceed under weakly acidic conditions utilizing a combination of two catalysts, an indoline HCl salt and a bisthiourea compound. Mechanistic investigations revealed the roles of both catalysts and confirmed the involvement of oxocarbenium ion intermediates, ruling out alternative scenarios. A stereochemical model was derived from density functional theory calculations, which provided the basis for the development of a highly enantioselective stereodivergent variant with racemic tryptophol derivatives.
Keywords: DFT calculations; anion-binding catalysis; asymmetric catalysis; dual catalysis; kinetic isotope effects; oxa-Pictet–Spengler reaction; oxocarbenium ions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Direct Formation of Oxocarbenium Ions under Weakly Acidic Conditions: Catalytic Enantioselective Oxa-Pictet-Spengler Reactions.J Am Chem Soc. 2016 Jul 27;138(29):9053-6. doi: 10.1021/jacs.6b05225. Epub 2016 Jul 13. J Am Chem Soc. 2016. PMID: 27396413
-
Exploring the Chemistry of Spiroindolenines by Mechanistically-Driven Reaction Development: Asymmetric Pictet-Spengler-type Reactions and Beyond.Acc Chem Res. 2020 Apr 21;53(4):974-987. doi: 10.1021/acs.accounts.0c00074. Epub 2020 Apr 10. Acc Chem Res. 2020. PMID: 32275392
-
Highly Acidic Conjugate-Base-Stabilized Carboxylic Acids Catalyze Enantioselective oxa-Pictet-Spengler Reactions with Ketals.Angew Chem Int Ed Engl. 2020 Jan 27;59(5):2028-2032. doi: 10.1002/anie.201912677. Epub 2019 Dec 10. Angew Chem Int Ed Engl. 2020. PMID: 31710767
-
Recent progress in the total synthesis of indole alkaloids.Curr Opin Drug Discov Devel. 2009 Nov;12(6):752-71. Curr Opin Drug Discov Devel. 2009. PMID: 19894188 Review.
-
[Research progress of Pictet-Spenglerases].Sheng Wu Gong Cheng Xue Bao. 2020 Oct 25;36(10):2001-2016. doi: 10.13345/j.cjb.200064. Sheng Wu Gong Cheng Xue Bao. 2020. PMID: 33169566 Review. Chinese.
Cited by
-
A genetic optimization strategy with generality in asymmetric organocatalysis as a primary target.Chem Sci. 2024 Jan 31;15(10):3640-3660. doi: 10.1039/d3sc06208b. eCollection 2024 Mar 6. Chem Sci. 2024. PMID: 38455002 Free PMC article.
-
Parallel kinetic resolution of aziridines via chiral phosphoric acid-catalyzed apparent hydrolytic ring-opening.Chem Sci. 2023 Oct 12;14(43):12152-12159. doi: 10.1039/d3sc03899h. eCollection 2023 Nov 8. Chem Sci. 2023. PMID: 37969581 Free PMC article.
References
-
-
Selected reviews on the oxa-Pictet–Spengler reaction:
- Larghi EL; Kaufman TS The oxa-Pictet-Spengler cyclization: Synthesis of isochromans and related pyran-type heterocycles. Synthesis 2006, 187–220.
- Larghi EL; Kaufman TS Synthesis of Oxacycles Employing the Oxa-Pictet–Spengler Reaction: Recent Developments and New Prospects. Eur. J. Org. Chem 2011, 2011, 5195–5231.
- Zhu Z; Adili A; Zhao C; Seidel D Catalytic Enantioselective Approaches to the oxa-Pictet–Spengler Cyclization and Other 3,6- Dihydropyran-Forming Reactions. SynOpen 2019, 03, 77–90.
-
-
- Buschmann H; Michel R Verfahren zur Herstellung von Isochromanen. German Patent 614461, 1935.
- Buschmann H; Michel R Verfahren zur Herstellung von Isochromanen, Zusatz. German Patent 617646, 1935.
-
- Doyle AG Engaging AlkyI Halides and Oxocarbenium Ions in Asymmetric Catalysis. Ph.D. Thesis, Harvard University: Cambridge, 2008, 267–293.
- Lombardo VM; Thomas CD; Scheidt KA A Tandem Isomerization/Prins Strategy: Iridium(III)/Brønsted Acid Cooperative Catalysis. Angew. Chem., Int. Ed 2013, 52, 12910–12914. - PMC - PubMed
- Ascic E; Ohm RG; Petersen R; Hansen MR; Hansen CL; Madsen D; Tanner D; Nielsen TE Synthesis of Oxacyclic Scaffolds via Dual Ruthenium Hydride/Brønsted Acid-Catalyzed Isomerization/Cyclization of Allylic Ethers. Chem. – Eur. J 2014, 20, 3297–3300. - PubMed
- Zhao C; Chen SB; Seidel D Direct Formation of Oxocarbenium Ions under Weakly Acidic Conditions: Catalytic Enantioselective Oxa-Pictet–Spengler Reactions. J. Am. Chem. Soc 2016, 138, 9053–9056. - PubMed
- Das S; Liu L; Zheng Y; Alachraf MW; Thiel W; De CK; List B Nitrated Confined Imidodiphosphates Enable a Catalytic Asymmetric Oxa-Pictet–Spengler Reaction. J. Am. Chem. Soc 2016, 138, 9429–9432. - PubMed
- Maskeri MA; O’Connor MJ; Jaworski AA; Davies AV; Scheidt KA A Cooperative Hydrogen Bond Donor–Brønsted Acid System for the Enantioselective Synthesis of Tetrahydropyrans. Angew. Chem., Int. Ed 2018, 57, 17225–17229. - PMC - PubMed
- Zhu Z; Odagi M; Zhao C; Abboud KA; Kirm HU; Saame J; Lõkov M; Leito I; Seidel D Highly Acidic Conjugate-Base-Stabilized Carboxylic Acids Catalyze Enantioselective oxa-Pictet–Spengler Reactions with Ketals. Angew. Chem., Int. Ed 2020, 59, 2028–2032. - PubMed
- Maskeri MA; Brueckner AC; Feoktistova T; O’Connor MJ; Walden DM; Cheong PH-Y; Scheidt KA Mechanism and origins of selectivity in the enantioselective oxa-Pictet–Spengler reaction: a cooperative catalytic complex from a hydrogen bond donor and chiral phosphoric acid. Chem. Sci 2020, 11, 8736–8743. - PMC - PubMed
- Riegel GF; Payne C; Kass SR Effects of Brønsted acid cocatalysts on the activities and selectivities of charge-enhanced thiourea organocatalysts in Friedel–Crafts and oxa-Pictet–Spengler reactions. J. Phys. Org. Chem 2022, No. e4321.
-
-
Selected recent reviews on the Pictet–Spengler reaction:
- Stöckigt J; Antonchick AP; Wu FR; Waldmann H The Pictet-Spengler Reaction in Nature and in Organic Chemistry. Angew. Chem., Int. Ed 2011, 50, 8538–8564. - PubMed
- Ingallina C; D’Acquarica I; Delle Monache G; Ghirga F; Quaglio D; Ghirga P; Berardozzi S; Markovic V; Botta B The Pictet-Spengler Reaction Still on Stage. Curr. Pharm. Des 2016, 22, 1808–1850. - PubMed
- Calcaterra A; Mangiardi L; Delle Monache G; Quaglio D; Balducci S; Berardozzi S; Iazzetti A; Franzini R; Botta B; Ghirga F The Pictet-Spengler Reaction Updates Its Habits. Molecules 2020, 25, 414. - PMC - PubMed
- Glinsky-Olivier N; Guinchard X Enantioselective Catalytic Methods for the Elaboration of Chiral Tetrahydro-β-carbolines and Related Scaffolds. Synthesis 2017, 49, 2605–2620.
- Gholamzadeh P Chapter Three - The Pictet–Spengler Reaction: A Powerful Strategy for the Synthesis of Heterocycles. In Advances in Heterocyclic Chemistry; Scriven EFV; Ramsden CA, Eds. Academic Press: 2019; Vol. 127, pp 153–226.
- Zheng C; You S-L Exploring the Chemistry of Spiroindolenines by Mechanistically-Driven Reaction Development: Asymmetric Pictet–Spengler-type Reactions and Beyond. Acc. Chem. Res 2020, 53, 974–987. - PubMed
-
-
-
Selected reviews covering aspects of catalytic enantioselective reactions involving oxocarbenium ions:
- Moyano A; Rios R Asymmetric Organocatalytic Cyclization and Cycloaddition Reactions. Chem. Rev 2011, 111, 4703–4832. - PubMed
- Gualandi A; Rodeghiero G; Cozzi PG Catalytic Stereoselective SN1-Type Reactions Promoted by Chiral Phosphoric Acids as Brønsted Acid Catalysts. Asian J. Org. Chem 2018, 7, 1957–1981.
- Tamanna; Kumar M; Joshi K; Chauhan P Catalytic Asymmetric Synthesis of Isochroman Derivatives. Adv. Synth. Catal 2020, 362, 1907–1926.
- Liu J; Dasgupta S; Watson MP Enantioselective additions of copper acetylides to cyclic iminium and oxocarbenium ions. Beilstein J. Org. Chem 2015, 11, 2696–2706. - PMC - PubMed
-
see also ref
- Lei C-W; Mu B-S; Zhou F; Yu J-S; Zhou Y; Zhou J Organocatalytic enantioselective reactions involving prochiral carbocationic intermediates. Chem. Commun 2021, 57, 9178–9191. - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
