Immunogenicity and safety of SARS-CoV-2 recombinant protein nanoparticle vaccine GBP510 adjuvanted with AS03: interim results of a randomised, active-controlled, observer-blinded, phase 3 trial
- PMID: 37711219
- PMCID: PMC10498190
- DOI: 10.1016/j.eclinm.2023.102140
Immunogenicity and safety of SARS-CoV-2 recombinant protein nanoparticle vaccine GBP510 adjuvanted with AS03: interim results of a randomised, active-controlled, observer-blinded, phase 3 trial
Abstract
Background: GBP510 vaccine contains self-assembling, recombinant nanoparticles displaying SARS-CoV-2 spike receptor-binding domains. We report interim phase 3 immunogenicity results for GBP510 adjuvanted with AS03 (GBP510/AS03) compared with ChAdOx1-S (Vaxzevria, AstraZeneca) in healthy adults aged ≥18 years, up to 6 months after the second dose.
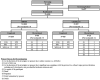
Methods: This was a randomised, active-controlled, observer-blinded, parallel group, phase 3 study, conducted at 38 sites across six countries (South Korea, Philippines, Thailand, Vietnam, Ukraine and New Zealand). Cohort 1 (no history of SARS-CoV-2 infection/COVID-19 vaccination) was randomised 2:1 to receive two doses of GBP510/AS03 or ChAdOx1-S (immunogenicity and safety), while Cohort 2 (regardless of baseline serostatus) was randomised 5:1 (safety). Primary objectives were to demonstrate superiority in geometric mean titre (GMT) and non-inferiority in seroconversion rate (SCR; ≥4-fold rise from baseline) of GBP510/AS03 vs. ChAdOx1-S for neutralising antibodies against the ancestral strain by live-virus neutralisation assay. Secondary objectives included assessment of safety and reactogenicity (long-term 6 months cut-off date: 09 August 2022). This study was registered on ClinicalTrials.gov (NCT05007951).
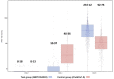
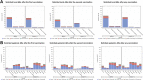
Findings: Between 30 August 2021 and 11 January 2022, a total of 4913 participants were screened and 4036 participants (1956 in Cohort 1 and 2080 in Cohort 2) who met eligibility criteria were enrolled and randomised to receive 2 doses of GBP510/AS03 (n = 3039) or ChAdOx1-S (n = 997). Most participants were Southeast Asian (81.5%) and aged 18-64 years (94.7%). The primary objectives assessed in per-protocol set included 877 participants in GBP510/AS03 and 441 in ChAdOx1-S group: at 2 weeks after the second vaccination, the GMT ratio (GBP510/AS03/ChAdOx1-S) in per-protocol set was 2.93 (95% CI 2.63-3.27), demonstrating superiority (95% CI lower limit >1) of GBP510/AS03; the between-group SCR difference of 10.8% (95% CI 7.68-14.32) also satisfied the non-inferiority criterion (95% CI lower limit > -5%). Neutralizing antibody titres sustained higher for the GBP510/AS03 group compared to the ChAdOx1-S group through 6 months after the second vaccination. In Safety analysis (Cohort 1 & 2), the proportion of participants with adverse events (AEs) after any vaccination was higher with GBP510/AS03 vs. ChAdOx1-S for solicited local AEs (56.7% vs. 49.2%), but was similar for solicited systemic AEs (51.2% vs. 53.5%) and unsolicited AEs (13.3% vs. 14.6%) up to 28 days after the second vaccination. No safety concerns were identified during follow-up for 6 months after the second vaccination.
Interpretation: Our interim findings suggested that GBP510/AS03 met the superiority criterion for neutralising antibodies and non-inferiority criterion for SCR compared with ChAdOx1-S, and showed a clinically acceptable safety profile.
Funding: This work was supported, in whole or in part, by funding from CEPI and the Bill & Melinda Gates Foundation Investments INV-010680 and INV-006462. The Bill & Melinda Gates Foundation supported this project for the generation of IND-enabling data and CEPI supported this clinical study.
Keywords: COVID-19; Immunogenicity; Nanoparticle vaccine; Recombinant protein vaccine; SARS-CoV-2; Safety.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
FR, TB, and PW are employees of the GSK group of companies. FR and TB hold restricted shares in the GSK group of companies. HK, JHR, SJL, and YWP are full-time employees of SK Bioscience. HK, YWP and JHR own SK Bioscience stock as employees. SS, MS and ND are full-time employees of IVI. All other authors declare no competing interests.
Figures
References
-
- Morel S., Didierlaurent A., Bourguignon P., et al. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

