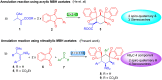
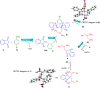
Multicomponent reaction (MCR) for constructing bis-spirocyclohexane skeletons using β-nitrostyrene derived MBH acetates, 1,3-indanedione and aldehydes via [1 + 1 + 1 + 3] annulation
- PMID: 37711377
- PMCID: PMC10498677
- DOI: 10.1039/d3ra04996e
Multicomponent reaction (MCR) for constructing bis-spirocyclohexane skeletons using β-nitrostyrene derived MBH acetates, 1,3-indanedione and aldehydes via [1 + 1 + 1 + 3] annulation
Abstract
An AB2C type four-component quadruple cascade reaction has been established between nitroallylic MBH acetate, 1,3-indanedione and aldehyde for generating bis-spirocyclohexanes. This reaction progressed in an unusual [1 + 1 + 1 + 3] annulation manner via a Knoevenagel/Michael/Michael/Michael sequence, resulting in the generation of three/four chiral centres, and two all-carbon quaternary centres through the formation of 3 new C-C bonds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
A triple cascade approach towards the diastereoselective synthesis of spiro trans-decalinol scaffolds.Chem Commun (Camb). 2022 Sep 15;58(74):10400-10403. doi: 10.1039/d2cc03562f. Chem Commun (Camb). 2022. PMID: 36039826
-
Pyridine-Catalyzed Chemoselective Four-Component Cascade Reaction of Aromatic Aldehydes, Malononitrile/Cyanoacetates, MBH Carbonates, and Alcohols.Org Lett. 2024 Sep 13;26(36):7576-7583. doi: 10.1021/acs.orglett.4c02612. Epub 2024 Sep 3. Org Lett. 2024. PMID: 39225685
-
One-pot regioselective synthesis of meta-terphenyls via [3 + 3] annulation of nitroallylic acetates with alkylidenemalononitriles.J Org Chem. 2014 Aug 15;79(16):7468-76. doi: 10.1021/jo501193h. Epub 2014 Jul 24. J Org Chem. 2014. PMID: 25032898
-
Morita-Baylis-Hillman (MBH) Reaction Derived Nitroallylic Alcohols, Acetates and Amines as Synthons in Organocatalysis and Heterocycle Synthesis.Chem Rec. 2017 Mar;17(3):363-381. doi: 10.1002/tcr.201600075. Epub 2016 Oct 4. Chem Rec. 2017. PMID: 27701816 Review.
-
Organocatalytic Synthesis of Highly Functionalized Heterocycles by Enantioselective aza-Morita-Baylis-Hillman-Type Domino Reactions.Chem Pharm Bull (Tokyo). 2020;68(4):299-315. doi: 10.1248/cpb.c19-00900. Chem Pharm Bull (Tokyo). 2020. PMID: 32238648 Review.
References
-
- Francke W. Kitching W. Curr. Org. Chem. 2001;5:233. doi: 10.2174/1385272013375652. - DOI
- Huang J. Frontier A. J. J. Am. Chem. Soc. 2007;129:8060. doi: 10.1021/ja0716148. - DOI - PubMed
- Companyo X. Zea A. Alba A. N. R. Mazzanti A. Moyano A. Rios R. Chem. Commun. 2010;46:6953. doi: 10.1039/C0CC01522A. - DOI - PubMed
- Rios R. Chem. Soc. Rev. 2012;41:1060. doi: 10.1039/C1CS15156H. - DOI - PubMed
-
- Ruijter E. Scheffelaar R. Orru R. V. A. Angew. Chem., Int. Ed. 2011;50:6234. doi: 10.1002/anie.201006515. - DOI - PubMed
- Domling A. Ugi I. Angew. Chem., Int. Ed. 2000;39:3168. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U. - DOI - PubMed
- Zhu J. and Bienayme H., Multicomponent Reactions, Wiley-VCH, Weinheim, Germany, 2005
- Tietze L. F. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. - DOI - PubMed
- Nicolaou K. C. Edmonds D. J. Bulger P. G. Angew. Chem., Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872. - DOI - PubMed
- Tietze L. F., Brasche G. and Gericke K. M., Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, Germany, 2006
- Basha S. F. Prasad T. N. Gudise V. B. Kumar V. S. Mulakayala N. Anwar S. Synth. Commun. 2019;49:3181. doi: 10.1080/00397911.2019.1659973. - DOI
-
- Yang Y. Wang X. Ye X. Wang B. Bao X. Wang H. Org. Biomol. Chem. 2021;19:4610. doi: 10.1039/D1OB00564B. - DOI - PubMed
- Boddy A. J. Bull J. A. Org. Chem. Front. 2021;8:1026. doi: 10.1039/D0QO01085E. - DOI
- Xu P.-W. Yu J.-S. Chen C. Cao Z.-Y. Zhou F. Zhou J. ACS Catal. 2019;9:1820. doi: 10.1021/acscatal.8b03694. - DOI
- Cheng Y.-S. Anwar S. Chen K. Mini-Rev. Org. Chem. 2018;15:364. doi: 10.2174/1570193X15666171228145726. - DOI
-
- Morita K.-i. Suzuki Z. Hirose H. Bull. Chem. Soc. Jpn. 1968;41:2815. doi: 10.1246/bcsj.41.2815. - DOI
- Baylis A. B. and Hillman M. E. D., German Patent DE 2155113, 1972
LinkOut - more resources
Full Text Sources
Miscellaneous