Amidinoquinoxaline N-oxides: synthesis and activity against anaerobic bacteria
- PMID: 37711381
- PMCID: PMC10498151
- DOI: 10.1039/d3ra01184d
Amidinoquinoxaline N-oxides: synthesis and activity against anaerobic bacteria
Abstract
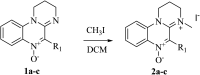
We present herein an in-depth study on the activity of amidinoquinoxaline N-oxides 1 against Gram-positive and Gram-negative anaerobic bacteria. Based on 5-phenyl-2,3-dihydropyrimidoquinoxaline N-oxide 1a, the selected structural variations included in our study comprise the substituents α- to the N-oxide function, the benzofused ring, substitution and quaternization of the amidine moiety, and the amidine ring size. Compounds 1 showed good to excellent antianaerobic activity, evaluated as the corresponding CIM50 and CIM90 values, and an antimicrobial spectrum similar to metronidazole. Six out of 13 compounds 1 had CIM90 values significantly lower than the reference drug. Among them, imidazoline derivatives 1i-l were the most active structures. Such compounds were synthesized by base-promoted ring closure of the corresponding amidines. The N-oxides under study showed no significant cytotoxicity against RAW 264.7 cells, with high selectivity indexes. Their calculated ADME properties indicate that the compounds are potentially good oral drug candidates. The antianaerobic activity correlated satisfactorily with the electron affinity of the compounds, suggesting that they may undergo bioreductive activation before exerting their antibacterial activity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

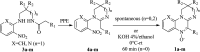
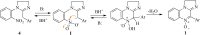

References
-
- Jousimies-Somer H., Summanen P., Citron D. M., Baron E. J., Wexler H. M. and Finegold S. M., Wadsworth-KTL Anaerobic Bacteriology Manual, Star, Corea, 6th edn, 2002
-
- Brook I., Antimicrobial Resistance of Anaerobic Bacteria, in Antimicrobial Drug Resistance, ed. D.L. Mayers, J. D. Sobel, M. Ouellette, K. S. Kaye and D. Marchaim, Springer International Publishing, 2017, p. 1007
-
- Löfmark S. Edlund C. Nor C. E. Clin. Infect. Dis. 2010;50:16. - PubMed
-
- Pence M. A. Clin. Microbiol. Newsl. 2019;41:1. - PMC - PubMed
- Fernández-Canigia L. Litterio M. Legaria M. C. Castello L. Predari S. C. Di Martino A. Rossetti A. Rollet R. Carloni G. Bianchini H. Cejas D. Radice M. Gutkind G. Anaerobe Surveillance Team. Antimicrob. Agents Chemother. 2012;56(3):1309. doi: 10.1128/AAC.05622-11. - DOI - PMC - PubMed
-
- Ingham H. R. Eaton S. Venables C. W. Adams P. C. Lancet. 1978;1:214. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

