Chemonucleolysis combined with dynamic loading for inducing degeneration in bovine caudal intervertebral discs
- PMID: 37711456
- PMCID: PMC10499327
- DOI: 10.3389/fbioe.2023.1178938
Chemonucleolysis combined with dynamic loading for inducing degeneration in bovine caudal intervertebral discs
Abstract
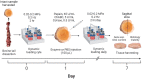
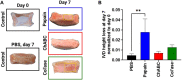
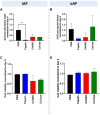
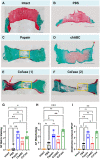
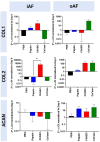
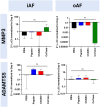
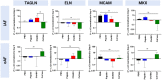
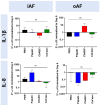
Chemonucleolysis has become an established method of producing whole organ culture models of intervertebral disc (IVD) degeneration. However, the field needs more side-by-side comparisons of the degenerative effects of the major enzymes used in chemonucleolysis towards gaining a greater understanding of how these organ culture models mimic the wide spectrum of characteristics observed in human degeneration. In the current work we induced chemonucleolysis in bovine coccygeal IVDs with 100 µL of papain (65 U/mL), chondroitinase ABC (chABC, 5 U/mL), or collagenase II (col'ase, 0.5 U/mL). Each enzyme was applied in a concentration projected to produce moderate levels of degeneration. After 7 days of culture with daily dynamic physiological loading (0.02-0.2 MPa, 0.2 Hz, 2 h), the cellular, biochemical and histological properties of the IVDs were evaluated in comparison to a PBS-injected control. Papain and collagenase, but not chABC, produced macroscopic voids in the tissues. Compared to day 0 intact IVDs, papain induced the greatest magnitude glycosaminoglycan (GAG) loss compared to chABC and col'ase. Papain also induced the greatest height loss (3%), compared to 0.7%, 1.2% and 0.4% for chABC, col'ase, and PBS, respectively. Cell viability in the region adjacent to papain and PBS-injection remained at nearly 100% over the 7-day culture period, whereas it was reduced to 60%-70% by chABC and col'ase. Generally, enzyme treatment tended to downregulate gene expression for major ECM markers, type I collagen (COL1), type II collagen (COL2), and aggrecan (ACAN) in the tissue adjacent to injection. However, chABC treatment induced an increase in COL2 gene expression, which was significant compared to the papain treated group. In general, papain and col'ase treatment tended to recapitulate aspects of advanced IVD degeneration, whereas chABC treatment captured aspects of early-stage degeneration. Chemonucleolysis of whole bovine IVDs is a useful tool providing researchers with a robust spectrum of degenerative changes and can be utilized for examination of therapeutic interventions.
Keywords: chemonucleolysis (CN); degeneration; extracellular matrix; intervertebral disc; organ culture.
Copyright © 2023 Vernengo, Bumann, Kluser, Soubrier, Šećerović, Gewiess, Jansen, Neidlinger-Wilke, Wilke and Grad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

