A cutting-edge strategy for spinal cord injury treatment: resident cellular transdifferentiation
- PMID: 37711511
- PMCID: PMC10498389
- DOI: 10.3389/fncel.2023.1237641
A cutting-edge strategy for spinal cord injury treatment: resident cellular transdifferentiation
Abstract
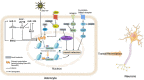
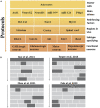
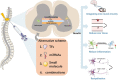
Spinal cord injury causes varying degrees of motor and sensory function loss. However, there are no effective treatments for spinal cord repair following an injury. Moreover, significant preclinical advances in bioengineering and regenerative medicine have not yet been translated into effective clinical therapies. The spinal cord's poor regenerative capacity makes repairing damaged and lost neurons a critical treatment step. Reprogramming-based neuronal transdifferentiation has recently shown great potential in repair and plasticity, as it can convert mature somatic cells into functional neurons for spinal cord injury repair in vitro and in vivo, effectively halting the progression of spinal cord injury and promoting functional improvement. However, the mechanisms of the neuronal transdifferentiation and the induced neuronal subtypes are not yet well understood. This review analyzes the mechanisms of resident cellular transdifferentiation based on a review of the relevant recent literature, describes different molecular approaches to obtain different neuronal subtypes, discusses the current challenges and improvement methods, and provides new ideas for exploring therapeutic approaches for spinal cord injury.
Keywords: direct reprogramming; nerve repair; neurons; spinal cord injury; transdifferentiation.
Copyright © 2023 Fang, Chen, Zheng, Yang, Zhang, Chen, Pei, Lin and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abernathy D. G., Kim W. K., McCoy M. J., Lake A. M., Ouwenga R., Lee S. W., et al. (2017). MicroRNAs induce a permissive chromatin environment that enables neuronal subtype-specific reprogramming of adult human fibroblasts. Cell Stem Cell 21 332–348.e9. 10.1016/j.stem.2017.08.002 - DOI - PMC - PubMed
-
- Aravantinou-Fatorou K., Ortega F., Chroni-Tzartou D., Antoniou N., Poulopoulou C., Politis P. K., et al. (2015). CEND1 and neurogenin2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursors and differentiated neurons. Stem Cell Rep. 5 405–418. 10.1016/j.stemcr.2015.07.012 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

