Murine model identifies tropomyosin as IgE cross-reactive protein between house dust mite and coho salmon that possibly contributes to the development of salmon allergy
- PMID: 37711608
- PMCID: PMC10498769
- DOI: 10.3389/fimmu.2023.1238297
Murine model identifies tropomyosin as IgE cross-reactive protein between house dust mite and coho salmon that possibly contributes to the development of salmon allergy
Abstract
Background: Recently, we have developed a method to identify IgE cross-reactive allergens. However, the mechanism by which IgE cross-reactive allergens cause food allergy is not yet fully understood how. In this study, we aimed to understand the underlying pathogenesis by identifying food allergens that cross-react with house dust mite allergens in a murine model.
Material and methods: Allergenic protein microarray analysis was conducted using serum from mice intraperitoneally injected with Dermatophagoides pteronyssinus (Der p) extract plus alum or alum alone as controls. Der p, Dermatophagoides farinae (Der f), coho salmon extract-sensitized and control mice were analyzed. Serum levels of IgE against Der p, Der f, coho salmon extract, protein fractions of coho salmon extract separated by ammonium sulfate precipitation and anion exchange chromatography, and recombinant coho salmon tropomyosin or actin were measured by an enzyme-linked immunosorbent assay. A murine model of cutaneous anaphylaxis or oral allergy syndrome (OAS) was established in Der p extract-sensitized mice stimulated with coho salmon extract, tropomyosin, or actin.
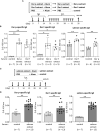
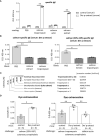
Results: Protein microarray analysis showed that coho salmon-derived proteins were highly bound to serum IgE in Der p extract-sensitized mice. Serum IgE from Der p or Der f extract-sensitized mice was bound to coho salmon extract, whereas serum IgE from coho salmon extract-sensitized mice was bound to Der p or Der f extract. Analysis of the murine model showed that cutaneous anaphylaxis and oral allergic reaction were evident in Der p extract-sensitized mice stimulated by coho salmon extract. Serum IgE from Der p or Der f extract-sensitized mice was bound strongly to protein fractions separated by anion exchange chromatography of coho salmon proteins precipitated with 50% ammonium sulfate, which massively contained the approximately 38 kDa protein. We found that serum IgE from Der p extract-sensitized mice was bound to recombinant coho salmon tropomyosin. Der p extract-sensitized mice exhibited cutaneous anaphylaxis in response to coho salmon tropomyosin.
Conclusion: Our results showed IgE cross-reactivity of tropomyosin between Dermatophagoides and coho salmon which illustrates salmon allergy following sensitization with the house dust mite Dermatophagoides. Our method for identifying IgE cross-reactive allergens will help understand the underlying mechanisms of food allergies.
Keywords: IgE cross-reactivity; food allergy; house dust mite; mast cell; murine model; salmon.
Copyright © 2023 Yamamoto, Izawa, Ando, Kaitani, Tanabe, Yamada, Uchida, Yoshikawa, Kume, Toriumi, Maehara, Wang, Nagamine, Negishi, Nakano, Ebihara, Shimizu, Ogawa, Okumura and Kitaura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

