Natural killer cells and BNT162b2 mRNA vaccine reactogenicity and durability
- PMID: 37711632
- PMCID: PMC10497936
- DOI: 10.3389/fimmu.2023.1225025
Natural killer cells and BNT162b2 mRNA vaccine reactogenicity and durability
Abstract
Introduction: Natural killer (NK) cells can both amplify and regulate immune responses to vaccination. Studies in humans and animals have observed NK cell activation within days after mRNA vaccination. In this study, we sought to determine if baseline NK cell frequencies, phenotype, or function correlate with antibody responses or inflammatory side effects induced by the Pfizer-BioNTech COVID-19 vaccine (BNT162b2).
Methods: We analyzed serum and peripheral blood mononuclear cells (PBMCs) from 188 participants in the Prospective Assessment of SARS-CoV-2 Seroconversion study, an observational study evaluating immune responses in healthcare workers. Baseline serum samples and PBMCs were collected from all participants prior to any SARS-CoV-2 infection or vaccination. Spike-specific IgG antibodies were quantified at one and six months post-vaccination by microsphere-based multiplex immunoassay. NK cell frequencies and phenotypes were assessed on pre-vaccination PBMCs from all participants by multi-color flow cytometry, and on a subset of participants at time points after the 1st and 2nd doses of BNT162b2. Inflammatory side effects were assessed by structured symptom questionnaires, and baseline NK cell functionality was quantified by an in vitro killing assay on participants that reported high or low post-vaccination symptom scores.
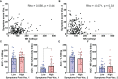
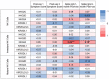
Results: Key observations include: 1) circulating NK cells exhibit evidence of activation in the week following vaccination, 2) individuals with high symptom scores after 1st vaccination had higher pre-vaccination NK cytotoxicity indices, 3) high pre-vaccination NK cell numbers were associated with lower spike-specific IgG levels six months after two BNT162b2 doses, and 4) expression of the inhibitory marker NKG2A on immature NK cells was associated with higher antibody responses 1 and 6 months post-vaccination.
Discussion: These results suggest that NK cell activation by BNT162b2 vaccination may contribute to vaccine-induced inflammatory symptoms and reduce durability of vaccine-induced antibody responses.
Keywords: COVID; NK cells; SARS-CoV-2 vaccine; antibody durability; mRNA vaccine; reactogenicity; vaccine side effects.
Copyright © 2023 Graydon, Conner, Dunham, Olsen, Goguet, Coggins, Rekedal, Samuels, Jackson-Thompson, Moser, Lindrose, Hollis-Perry, Wang, Maiolatesi, Alcorta, Reyes, Wong, Ramsey, Davies, Parmelee, Ortega, Sanchez, Moller, Inglefield, Tribble, Burgess, O’Connell, Malloy, Pollett, Broder, Laing, Anderson and Mitre.
Conflict of interest statement
SP, TB, and DT report that the Uniformed Services University USU Infectious Disease Clinical Research Program IDCRP, a US Department of Defense institution, and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. HJF were funded under a Cooperative Research and Development Agreement to conduct an unrelated phase III COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase III vaccine trial sponsored by AstraZeneca. Both trials were part of the US Government COVID-19 response. Neither is related to the work presented here. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Centers for Disease Control and Prevention . COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC. (2023) Available at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-boost....
-
- Centers for Disease Control . Pfizer-BioNTech COVID-19 Vaccine Reactions & Adverse Events (2022). Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenic....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

