Transition-metal (oxy)nitride photocatalysts for water splitting
- PMID: 37712021
- PMCID: PMC10498681
- DOI: 10.1039/d3sc03198e
Transition-metal (oxy)nitride photocatalysts for water splitting
Abstract
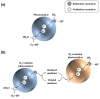
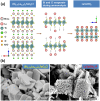
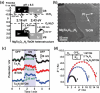
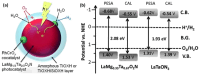
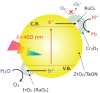
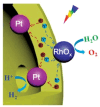
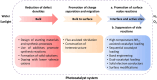
Solar-driven water splitting based on particulate semiconductor materials is studied as a technology for green hydrogen production. Transition-metal (oxy)nitride photocatalysts are promising materials for overall water splitting (OWS) via a one- or two-step excitation process because their band structure is suitable for water splitting under visible light. Yet, these materials suffer from low solar-to-hydrogen energy conversion efficiency (STH), mainly because of their high defect density, low charge separation and migration efficiency, sluggish surface redox reactions, and/or side reactions. Their poor thermal stability in air and under the harsh nitridation conditions required to synthesize these materials makes further material improvements difficult. Here, we review key challenges in the two different OWS systems and highlight some strategies recently identified as promising for improving photocatalytic activity. Finally, we discuss opportunities and challenges facing the future development of transition-metal (oxy)nitride-based OWS systems.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Segev G. Kibsgaard J. Hahn C. Xu Z. J. Cheng W. H. Deutsch T. G. Xiang C. X. Zhang J. Z. Hammarstrom L. Nocera D. G. Weber A. Z. Agbo P. Hisatomi T. Osterloh F. E. Domen K. Abdi F. F. Haussener S. Miller D. J. Ardo S. McIntyre P. C. Hannappel T. Hu S. Atwater H. Gregoire J. M. Ertem M. Z. Sharp I. D. Choi K. S. Lee J. S. Ishitani O. Ager J. W. Prabhakar R. R. Bell A. T. Boettcher S. W. Vincent K. Takanabe K. Artero V. Napier R. Roldan Cuenya B. Koper M. T. M. Van de Krol R. Houle F. J. Phys. D: Appl. Phys. 2022;55:323003. doi: 10.1088/1361-6463/ac6f97. - DOI
-
- Hisatomi T. Domen K. Nat. Catal. 2019;2:387–399. doi: 10.1038/s41929-019-0242-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

