Comparison of fine-needle aspiration techniques
- PMID: 37712216
- PMCID: PMC10872887
- DOI: 10.1111/jmp.12676
Comparison of fine-needle aspiration techniques
Abstract
Background: Fine-needle aspiration (FNA) has been reported since 1912 beginning with the use of trocars and other specialized instruments that were impractical. Since then, FNA has proven to be a successful alternative technique to excisional biopsy for some assays despite a few limitations.
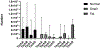
Methods: In this study, we compared four different techniques for FNA in rhesus macaques by evaluating total live cells recovered and cell viability using a standard 6 mL syringe and 1.5-inch 22-gauge needle.
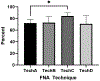
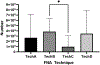
Results: Technique B which was the only technique in which the needle was removed from the syringe after collection of the sample to allow forced air through the needle to expel the contents into media followed by flushing of the syringe and needle resulted in the highest total cell count and second highest cell viability in recovered cells.
Conclusion: Based on our results, Technique B appears to be the superior method.
Keywords: biopsy; cell viability; fine-needle aspiration; lymph node; needle core biopsy; total cell count.
© 2023 The Authors. Journal of Medical Primatology published by John Wiley & Sons Ltd.
Figures




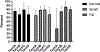
References
-
- Bart PA, Meuwly JY, Corpataux JM, Yerly S, Rizzardi P, Fleury S, Munoz M, Knabenhans C, Welbon C, Pantaleo G and Meylan PR (1999). “Sampling lymphoid tissue cells by ultrasound-guided fine needle aspiration of lymph nodes in HIV-infected patients. Swiss HIV Cohort Study.” AIDS 13(12): 1503–1509. - PubMed
-
- Boyd JD, Smith GD, Hong H, Mageau R and Juskevicius R (2015). “Fine-needle aspiration is superior to needle core biopsy as a sample acquisition method for flow cytometric analysis in suspected hematologic neoplasms.” Cytometry B Clin Cytom 88(1): 64–68. - PubMed
-
- Burgisser P, et al., Monitoring responses to antiretroviral treatment in human immunodeficiency virus type 1 (HIV-1)-infected patients by serial lymph node aspiration. J Infect Dis, 1997. 175(5): p. 1202–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

