Cardiac macrophages and emerging roles for their metabolism after myocardial infarction
- PMID: 37712418
- PMCID: PMC10503791
- DOI: 10.1172/JCI171953
Cardiac macrophages and emerging roles for their metabolism after myocardial infarction
Abstract
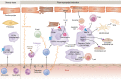
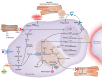
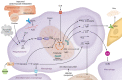
Interest in cardioimmunology has reached new heights as the experimental cardiology field works to tap the unrealized potential of immunotherapy for clinical care. Within this space is the cardiac macrophage, a key modulator of cardiac function in health and disease. After a myocardial infarction, myeloid macrophages both protect and harm the heart. To varying degrees, such outcomes are a function of myeloid ontogeny and heterogeneity, as well as functional cellular plasticity. Diversity is further shaped by the extracellular milieu, which fluctuates considerably after coronary occlusion. Ischemic limitation of nutrients constrains the metabolic potential of immune cells, and accumulating evidence supports a paradigm whereby macrophage metabolism is coupled to divergent inflammatory consequences, although experimental evidence for this in the heart is just emerging. Herein we examine the heterogeneous cardiac macrophage response following ischemic injury, with a focus on integrating putative contributions of immunometabolism and implications for therapeutically relevant cardiac injury versus cardiac repair.
Conflict of interest statement
Figures
Similar articles
-
Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury.Circ Res. 2019 Jan 18;124(2):263-278. doi: 10.1161/CIRCRESAHA.118.314028. Circ Res. 2019. PMID: 30582448 Free PMC article.
-
Macrophages of the Heart: Homeostasis and Disease.Biomed J. 2025 Apr 27:100867. doi: 10.1016/j.bj.2025.100867. Online ahead of print. Biomed J. 2025. PMID: 40300670
-
Lgr4 Governs a Pro-Inflammatory Program in Macrophages to Antagonize Post-Infarction Cardiac Repair.Circ Res. 2020 Sep 25;127(8):953-973. doi: 10.1161/CIRCRESAHA.119.315807. Epub 2020 Jun 30. Circ Res. 2020. PMID: 32600176
-
Effects of IL-38 on Macrophages and Myocardial Ischemic Injury.Front Immunol. 2022 May 13;13:894002. doi: 10.3389/fimmu.2022.894002. eCollection 2022. Front Immunol. 2022. PMID: 35634320 Free PMC article. Review.
-
The Role of Innate Immune Cells in Cardiac Injury and Repair: A Metabolic Perspective.Curr Cardiol Rep. 2023 Jul;25(7):631-640. doi: 10.1007/s11886-023-01897-4. Epub 2023 May 30. Curr Cardiol Rep. 2023. PMID: 37249739 Free PMC article. Review.
Cited by
-
Human and gut microbiota synergy in a metabolically active superorganism: a cardiovascular perspective.Front Cardiovasc Med. 2024 Oct 11;11:1411306. doi: 10.3389/fcvm.2024.1411306. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39465131 Free PMC article. Review.
-
Comprehensive macro and micro views on immune cells in ischemic heart disease.Cell Prolif. 2024 Dec;57(12):e13725. doi: 10.1111/cpr.13725. Epub 2024 Aug 1. Cell Prolif. 2024. PMID: 39087342 Free PMC article. Review.
-
Macrophage plasticity: signaling pathways, tissue repair, and regeneration.MedComm (2020). 2024 Aug 1;5(8):e658. doi: 10.1002/mco2.658. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39092292 Free PMC article. Review.
-
Cardiac macrophage metabolism in health and disease.Trends Endocrinol Metab. 2024 Mar;35(3):249-262. doi: 10.1016/j.tem.2023.10.011. Epub 2023 Nov 21. Trends Endocrinol Metab. 2024. PMID: 37993313 Free PMC article. Review.
-
Cardiac metabolism in the elderly: effects and consequences.Aging (Albany NY). 2024 Aug 19;16(16):11773-11775. doi: 10.18632/aging.206071. Epub 2024 Aug 19. Aging (Albany NY). 2024. PMID: 39167437 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

