Beyond epithelial damage: vascular and endothelial contributions to idiopathic pulmonary fibrosis
- PMID: 37712420
- PMCID: PMC10503802
- DOI: 10.1172/JCI172058
Beyond epithelial damage: vascular and endothelial contributions to idiopathic pulmonary fibrosis
Abstract
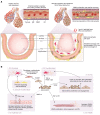
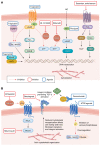
Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disease of the lung with poor survival. The incidence and mortality of IPF are rising, but treatment remains limited. Currently, two drugs can slow the scarring process but often at the expense of intolerable side effects, and without substantially changing overall survival. A better understanding of mechanisms underlying IPF is likely to lead to improved therapies. The current paradigm proposes that repetitive alveolar epithelial injury from noxious stimuli in a genetically primed individual is followed by abnormal wound healing, including aberrant activity of extracellular matrix-secreting cells, with resultant tissue fibrosis and parenchymal damage. However, this may underplay the importance of the vascular contribution to fibrogenesis. The lungs receive 100% of the cardiac output, and vascular abnormalities in IPF include (a) heterogeneous vessel formation throughout fibrotic lung, including the development of abnormal dilated vessels and anastomoses; (b) abnormal spatially distributed populations of endothelial cells (ECs); (c) dysregulation of endothelial protective pathways such as prostacyclin signaling; and (d) an increased frequency of common vascular and metabolic comorbidities. Here, we propose that vascular and EC abnormalities are both causal and consequential in the pathobiology of IPF and that fuller evaluation of dysregulated pathways may lead to effective therapies and a cure for this devastating disease.
Conflict of interest statement
Figures