Cytokine hemoadsorption with CytoSorb® in patients with sepsis: a systematic review and meta-analysis
- PMID: 37712812
- PMCID: PMC10406402
- DOI: 10.5935/2965-2774.20230289-en
Cytokine hemoadsorption with CytoSorb® in patients with sepsis: a systematic review and meta-analysis
Abstract
Objective: To analyze the effect of CytoSorb® on mortality, interleukin levels, vasopressor use and adverse events in patients with sepsis.
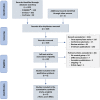
Methods: We searched MEDLINE®, Embase and the Cochrane Library for randomized controlled trials and cohort studies that reported the use of CytoSorb® among septic patients. The primary outcome was mortality, and secondary outcomes included the use of vasopressors, levels of inflammatory markers, predicted versus observed mortality, length of stay in the intensive care unit, and adverse events.
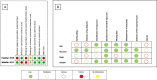
Results: We included 6 studies enrolling 413 patients, and assessment for risk of bias indicated variations in study quality from high to moderate. The overall mortality rate was 45%, and no significant effect on mortality was found at 28 - 30 days (RR 0.98 [0.12 - 8.25] for the randomized clinical trial and RR 0.74 [0.49 - 1.13] for cohort studies). We did not perform a metanalysis for other outcomes due to the small number of studies found or the lack of data.
Conclusion: Our study found very low certainty evidence, due to imprecision, risk of bias, and heterogeneity, thereby showing no benefit of CytoSorb® use in terms of mortality at 28 - 30 days. We cannot recommend the use of CytoSorb® in septic or septic shock patients outside clinical trials. Further high-quality randomized trials with a common intervention arm are needed to evaluate the influence of CytoSorb® in this population.
Prospero register: CRD42021262219.
Figures


References
-
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–143. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

