'Immunization during ART and ATI for HIV-1 vaccine discovery/development'
- PMID: 37712859
- PMCID: PMC10552831
- DOI: 10.1097/COH.0000000000000817
'Immunization during ART and ATI for HIV-1 vaccine discovery/development'
Abstract
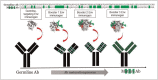
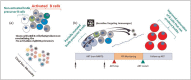
Purpose of review: Explore whether immunization with germline-targeting Env immunogens during ART, followed by ATI, leads to the identification of viral envelope glycoproteins (Envs) that promote and guide the full maturation of broadly neutralizing antibody responses.
Recent findings: The HIV-1 envelope glycoprotein (Env) does not efficiently engage the germline precursors of broadly neutralizing antibodies (bnAbs). However, Env-derived proteins specifically designed to precisely do that, have been recently developed. These 'germline-targeting' Env immunogens activate naïve B cells that express the germline precursors of bnAbs but by themselves cannot guide their maturation towards their broadly neutralizing forms. This requires sequential immunizations with heterologous sets of Envs. These 'booster' Envs are currently unknown.
Summary: Combining germline-targeting Env immunization approaches during ART with ATI could lead to the identification of natural Envs that are responsible for the maturation of broadly neutralizing antibody responses during infection. Such Envs could then serve as booster immunogens to guide the maturation of glBCRs that have become activated by germline-targeting immunogens in uninfected subjects.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures

Similar articles
-
Inducible cell lines producing replication-defective human immunodeficiency virus particles containing envelope glycoproteins stabilized in a pretriggered conformation.J Virol. 2024 Dec 17;98(12):e0172024. doi: 10.1128/jvi.01720-24. Epub 2024 Nov 7. J Virol. 2024. PMID: 39508605 Free PMC article.
-
Increased immunogen valency improves the maturation of vaccine-elicited HIV-1 VRC01-like antibodies.PLoS Pathog. 2025 May 29;21(5):e1013039. doi: 10.1371/journal.ppat.1013039. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40440315 Free PMC article.
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
-
Progress with induction of HIV broadly neutralizing antibodies in the Duke Consortia for HIV/AIDS Vaccine Development.Curr Opin HIV AIDS. 2023 Nov 1;18(6):300-308. doi: 10.1097/COH.0000000000000820. Epub 2023 Sep 25. Curr Opin HIV AIDS. 2023. PMID: 37751363 Free PMC article. Review.
-
Guiding HIV-1 vaccine development with preclinical nonhuman primate research.Curr Opin HIV AIDS. 2023 Nov 1;18(6):315-322. doi: 10.1097/COH.0000000000000819. Epub 2023 Sep 11. Curr Opin HIV AIDS. 2023. PMID: 37712825 Free PMC article. Review.
Cited by
-
HIV Vaccine Development at a Crossroads: New B and T Cell Approaches.Vaccines (Basel). 2024 Sep 12;12(9):1043. doi: 10.3390/vaccines12091043. Vaccines (Basel). 2024. PMID: 39340073 Free PMC article. Review.
-
Human Immunodeficiency Virus Vaccine: Promise and Challenges.Infect Dis Clin North Am. 2024 Sep;38(3):475-485. doi: 10.1016/j.idc.2024.04.004. Epub 2024 Jun 13. Infect Dis Clin North Am. 2024. PMID: 38876903 Review.
References
-
- Esmaeilzadeh E, Etemad B, Lavine CL, et al. . Autologous neutralizing antibodies increase with early antiretroviral therapy and shape HIV rebound after treatment interruption. Sci Transl Med 2023; 15:eabq4490. - PMC - PubMed
-
This is a comprehensive analysis of the evolution of both the viral Env and the autologous antibody responses prior and during ATI.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

