Discovery medicine - the HVTN's iterative approach to developing an HIV-1 broadly neutralizing vaccine
- PMID: 37712873
- PMCID: PMC10552837
- DOI: 10.1097/COH.0000000000000821
Discovery medicine - the HVTN's iterative approach to developing an HIV-1 broadly neutralizing vaccine
Abstract
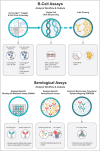
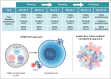
Purpose of review: In the past two decades, there has been an explosion in the discovery of HIV-1 broadly neutralizing antibodies (bnAbs) and associated vaccine strategies to induce them. This abundance of approaches necessitates a system that accurately and expeditiously identifies the most promising regimens. We herein briefly review the background science of bnAbs, provide a description of the first round of phase 1 discovery medicine studies, and suggest an approach to integrate these into a comprehensive HIV-1-neutralizing vaccine.
Recent findings: With recent preclinical success including induction of early stage bnAbs in mouse knockin models and rhesus macaques, successful priming of VRC01-class bnAbs with eOD-GT8 in a recent study in humans, and proof-of-concept that intravenous infusion of VRC01 prevents sexual transmission of virus in humans, the stage is set for a broad and comprehensive bnAb vaccine program. Leveraging significant advances in protein nanoparticle science, mRNA technology, adjuvant development, and B-cell and antibody analyses, the HVTN has reconfigured its HIV-1 vaccine strategy by developing the Discovery Medicine Program to test promising vaccine candidates targeting six key epitopes.
Summary: The HVTN Discovery Medicine program is testing multiple HIV-1-neutralizing vaccine candidates.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Figures


References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. . Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–2220. - PubMed
-
- Saphire EO, Parren PW, Pantophlet R, et al. . Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 2001; 293:1155–1159. - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, et al. . HIV vaccine design and the neutralizing antibody problem. Nat Immunol 2004; 5:233–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

