A Phase I Study of the Pan-Notch Inhibitor CB-103 for Patients with Advanced Adenoid Cystic Carcinoma and Other Tumors
- PMID: 37712875
- PMCID: PMC10501326
- DOI: 10.1158/2767-9764.CRC-23-0333
A Phase I Study of the Pan-Notch Inhibitor CB-103 for Patients with Advanced Adenoid Cystic Carcinoma and Other Tumors
Abstract
Purpose: CB-103 selectively inhibits the CSL-NICD (Notch intracellular domain) interaction leading to transcriptional downregulation of oncogenic Notch pathway activation. This dose-escalation/expansion study aimed to determine safety, pharmacokinetics, and preliminary antitumor activity.
Experimental design: Patients ≥18 years of age with selected advanced solid tumors [namely, adenoid cystic carcinoma (ACC)] and hematologic malignancies were eligible. CB-103 was dosed orally in cycles of 28 days at escalating doses until disease progression. Notch-activating mutations were required in a dose confirmatory cohort. Endpoints included dose-limiting toxicities (DLT), safety, tumor response, pharmacokinetics, and pharmacodynamics. Exploratory analyses focused on correlates of Notch and target gene expression.
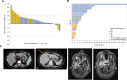
Results: Seventy-nine patients (64, 12 dose-escalation cohorts; 15, confirmatory cohort) enrolled with 54% receiving two or more lines of prior therapy. ACC was the dominant tumor type (40, 51%). Two DLTs were observed [elevated gamma-glutamyl transferase (GGT), visual change]; recommended phase II dose was declared as 500 mg twice daily (5 days on, 2 days off weekly). Grade 3-4 treatment-related adverse events occurred in 15 patients (19%), including elevated liver function tests (LFTs), anemia, and visual changes. Five (6%) discontinued drug for toxicity; with no drug-related deaths. There were no objective responses, but 37 (49%) had stable disease; including 23 of 40 (58%) patients with ACC. In the ACC cohort, median progression-free survival was 2.5 months [95% confidence interval (CI), 1.5-3.7] and median overall survival was 18.4 months (95% CI, 6.3-not reached).
Conclusions: CB-103 had a manageable safety profile and biological activity but limited clinical antitumor activity as monotherapy in this first-in-human study.
Significance: CB-103 is a novel oral pan-Notch inhibitor that selectively blocks the CSL-NICD interaction leading to transcriptional downregulation of oncogenic Notch pathway activation. This first-in-human dose-escalation and -confirmation study aimed to determine the safety, pharmacokinetics, and preliminary antitumor efficacy of CB-103. We observed a favorable safety profile with good tolerability and biological activity but limited clinical single-agent antitumor activity. Some disease stabilization was observed among an aggressive NOTCH-mutant ACC type-I subgroup where prognosis is poor and therapies are critically needed. Peripheral downregulation of select Notch target gene levels was observed with escalating doses. Future studies exploring CB-103 should enrich for patients with NOTCH-mutant ACC and investigate rational combinatorial approaches in tumors where there is limited success with investigational or approved drugs.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene 2008;27:5099–109. - PubMed
-
- Reynolds TC, Smith SD, Sklar J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell 1987;50:107–17. - PubMed
-
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011;11:338–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

