A missense mutation in zinc finger homeobox-3 (ZFHX3) impedes growth and alters metabolism and hypothalamic gene expression in mice
- PMID: 37713040
- PMCID: PMC7615594
- DOI: 10.1096/fj.202201829R
A missense mutation in zinc finger homeobox-3 (ZFHX3) impedes growth and alters metabolism and hypothalamic gene expression in mice
Abstract
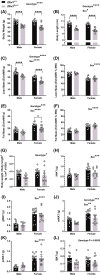
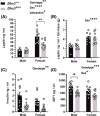
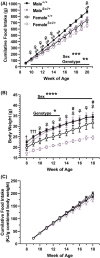
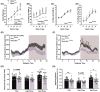
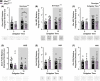
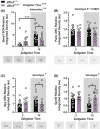
A protein altering variant in the gene encoding zinc finger homeobox-3 (ZFHX3) has recently been associated with lower BMI in a human genome-wide association study. We investigated metabolic parameters in mice harboring a missense mutation in Zfhx3 (Zfhx3Sci/+ ) and looked for altered in situ expression of transcripts that are associated with energy balance in the hypothalamus to understand how ZFHX3 may influence growth and metabolic effects. One-year-old male and female Zfhx3Sci/+ mice weighed less, had shorter body length, lower fat mass, smaller mesenteric fat depots, and lower circulating insulin, leptin, and insulin-like growth factor-1 (IGF1) concentrations than Zfhx3+/+ littermates. In a second cohort of 9-20-week-old males and females, Zfhx3Sci/+ mice ate less than wildtype controls, in proportion to body weight. In a third cohort of female-only Zfhx3Sci/+ and Zfhx3+/+ mice that underwent metabolic phenotyping from 6 to 14 weeks old, Zfhx3Sci/+ mice weighed less and had lower lean mass and energy expenditure, but fat mass did not differ. We detected increased expression of somatostatin and decreased expression of growth hormone-releasing hormone and growth hormone-receptor mRNAs in the arcuate nucleus (ARC). Similarly, ARC expression of orexigenic neuropeptide Y was decreased and ventricular ependymal expression of orphan G protein-coupled receptor Gpr50 was decreased. We demonstrate for the first time an energy balance effect of the Zfhx3Sci mutation, likely by altering expression of key ARC neuropeptides to alter growth, food intake, and energy expenditure.
Keywords: Gpr50; IGF1; appetite; atbf1; energy balance; growth; mouse model; somatostatin; zfhx3.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures
References
-
- Akiyama M, Okada Y, Kanai M, et al. Genome‐wide association study identifies 112 new loci for body mass index in the Japanese population. Nat Genet. 2017;49:1458‐1467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

