Living Material with Temperature-Dependent Light Absorption
- PMID: 37713073
- PMCID: PMC10602556
- DOI: 10.1002/advs.202301730
Living Material with Temperature-Dependent Light Absorption
Abstract
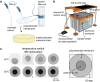
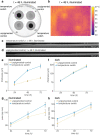
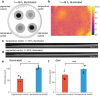
Engineered living materials (ELMs) exhibit desirable characteristics of the living component, including growth and repair, and responsiveness to external stimuli. Escherichia coli (E. coli) are a promising constituent of ELMs because they are very tractable to genetic engineering, produce heterologous proteins readily, and grow exponentially. However, seasonal variation in ambient temperature presents a challenge in deploying ELMs outside of a laboratory environment because E. coli growth rate is impaired both below and above 37 °C. Here, a genetic circuit is developed that controls the expression of a light-absorptive chromophore in response to changes in temperature. It is demonstrated that at temperatures below 36 °C, the engineered E. coli increase in pigmentation, causing an increase in sample temperature and growth rate above non-pigmented counterparts in a model planar ELM. On the other hand, at above 36 °C, they decrease in pigmentation, protecting the growth compared to bacteria with temperature-independent high pigmentation. Integrating the temperature-responsive circuit into an ELM has the potential to improve living material performance by optimizing growth and protein production in the face of seasonal temperature changes.
Keywords: engineered living materials; synthetic biology; thermal control.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gilbert C., Ellis T., ACS Synth. Biol. 2019, 8, 1. - PubMed
-
- Liu S., Xu W., Front. Sens. 2020, 1.
-
- Heveran C. M., Williams S. L., Qiu J., Artier J., Hubler M. H., Cook S. M., Cameron J. C., Srubar W. V., Matter 2020, 2, 481.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
