Efficacy of Guselkumab in Treating Nails, Scalp, Hands, and Feet in Patients with Psoriasis and Self-reported Psoriatic Arthritis
- PMID: 37713133
- PMCID: PMC10613182
- DOI: 10.1007/s13555-023-01012-z
Efficacy of Guselkumab in Treating Nails, Scalp, Hands, and Feet in Patients with Psoriasis and Self-reported Psoriatic Arthritis
Abstract
Introduction: The aim of this study was to evaluate guselkumab efficacy on regional psoriasis in a subset of psoriasis patients with a self-reported psoriatic arthritis (PsA) diagnosis.
Methods: In the phase 3 VOYAGE-1 and -2 studies, at week (W)0, patients with moderate-to-severe psoriasis were randomized to guselkumab 100 mg, placebo → guselkumab 100 mg at W16 through W44, or adalimumab 80 mg then 40 mg at W1 through W48 (VOYAGE-1) or W24 (VOYAGE-2). Pooled efficacy outcomes, including scalp-specific Investigator's Global Assessment (ss-IGA), hands and/or feet Physician's Global Assessment (hf-PGA), fingernail PGA (f-PGA), Nail Psoriasis Area and Severity Index (NAPSI), and Dermatology Life Quality Index (DLQI), were compared (nominal p-values) through W24 in patients with self-reported PsA diagnosis. Response rates/percentage improvement from baseline were determined, employing treatment failure rules and non-response/no improvement data imputation.
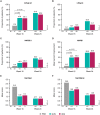
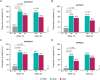
Results: A total of 76, 153, and 106 psoriasis patients with self-reported PsA were randomized to the placebo, guselkumab, or adalimumab groups, respectively; the baseline characteristics of patients in all three arms were comparable. At W16, a greater proportion of guselkumab- versus placebo-treated patients achieved ss-IGA 0/1 (80.6% vs. 22.7%, p < 0.001), hf-PGA 0/1 (68.9% vs. 14.8%, p < 0.001), f-PGA 0/1 (47.6% vs. 17.0%, p < 0.001), and DLQI 0/1 (45.6% vs. 2.7%, p < 0.001) responses; mean percentage NAPSI improvement was also greater with guselkumab (39.5% vs. 6.5%, p < 0.001). At W24, patients receiving guselkumab had higher ss-IGA 0/1 (77.5% vs. 58.5%, p = 0.003) and DLQI 0/1 (47.7% vs. 34.3%, p = 0.024) response rates versus those receiving adalimumab. Response rates/mean percentage improvements at W48 (VOYAGE-1) were numerically greater with guselkumab than adalimumab (e.g., NAPSI improvement: 75.6% vs. 60.9%).
Conclusions: Guselkumab-treated patients with psoriasis and self-reported PsA showed meaningful improvements in nail, scalp, and palmoplantar psoriasis.
Trial registration: VOYAGE-1 (ClinicalTrials.gov Identifier: NCT02207231) and VOYAGE-2 (ClinicalTrials.gov Identifier: NCT02207244).
Keywords: Guselkumab; Nail psoriasis; Palmoplantar psoriasis; Psoriatic arthritis; Scalp psoriasis.
© 2023. The Author(s).
Conflict of interest statement
Ana-Maria Orbai has received grant/research support from AbbVie, Amgen, Celgene, Eli Lilly, Horizon, Novartis, and Janssen; and consulting fees from Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Soumya D Chakravarty is an employee of Janssen Scientific Affairs, LLC and owns stock or stock options in Johnson & Johnson. Yin You is an employee of Janssen Research & Development, LLC. Ya-Wen Yang is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson. May Shawi is an employee of Janssen Research & Development, LLC, and owns stock in Johnson & Johnson. Joseph F Merola is a consultant and/or investigator for AbbVie, Amgen, Biogen, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Janssen, Leo Pharma, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB.
Figures

References
-
- Tremfya: package insert. Horsham: Janssen Biotech, Inc.; 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribi....
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

