Characteristics of Complement Protein Deposition in Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposition
- PMID: 37713183
- PMCID: PMC10723927
- DOI: 10.2215/CJN.0000000000000295
Characteristics of Complement Protein Deposition in Proliferative Glomerulonephritis with Monoclonal Immunoglobulin Deposition
Abstract
Background: Hypocomplementemia and complement co-deposition with monoclonal immunoglobulins in glomeruli are not rare in proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID). Deposition of monoclonal immunoglobulins in glomeruli has been suggested to activate complement and cause kidney injury. However, the profiles of complement activation in PGNMID and their clinical and pathologic significance need to be clarified.
Methods: Forty-six patients with PGNMID were enrolled. Proteomic analysis of glomeruli using laser microdissection and mass spectrometry was performed for ten patients with PGNMID to determine the composition of glomerular deposits. Kidney deposition of complement components was detected by immunohistochemistry and immunofluorescence. Urinary and plasma levels of complement components were measured by an enzyme-linked immunosorbent assay. Group differences were assessed using t tests or Mann-Whitney U tests depending on the distribution. Correlation analysis was performed using Spearman rank correlation or Pearson correlation.
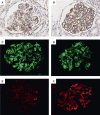
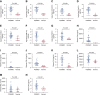
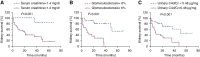
Results: Laser microdissection and mass spectrometry-based proteomic analysis showed that complement components were the most enriched proteins deposited in the glomeruli of patients with PGNMID. Glomerular deposition of C3c, C4d, and C5b-9 was detected in most patients. Levels of urinary and plasma C3a, C5a, soluble C5b-9, C4d, Bb, and C1q as well as urinary mannose-binding lectin were significantly higher in patients with PGNMID compared with healthy controls. The intensity of C3c and C4d deposition in glomeruli correlated with serum creatinine and the percentage of crescents, respectively. Furthermore, levels of urinary complement components correlated positively with serum creatinine, urinary protein excretion, percentage of crescents, and global glomerulosclerosis in kidney biopsies, whereas plasma levels of most complement components did not show a significant correlation with clinicopathologic parameters. In multivariable analysis, a higher level of urinary C4d was identified as an independent risk factor of kidney failure.
Conclusions: The complement system was found to be overactivated in PGNMID, and levels of urinary complements correlated with disease severity. A higher level of urinary C4d was identified as an independent risk factor of kidney failure.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
M.-h. Zhao reports consultancy for AstraZeneca, GSK, INFLARX, Kira, Novartis, and Roche. All remaining authors have nothing to disclose.
Figures


Similar articles
-
IgM Variant of Proliferative Glomerulonephritis With Monoclonal Immunoglobulin Deposits: A Case Series.Am J Kidney Dis. 2025 Jun;85(6):716-726.e1. doi: 10.1053/j.ajkd.2025.01.009. Epub 2025 Mar 25. Am J Kidney Dis. 2025. PMID: 40147751
-
Proliferative glomerulonephritis with monoclonal immunoglobulin deposits: an entity associated with distinct diseases and comparison between IgG1 and IgG3 subtypes.J Nephrol. 2022 Dec;35(9):2363-2372. doi: 10.1007/s40620-022-01317-w. Epub 2022 Apr 23. J Nephrol. 2022. PMID: 35460458
-
Complement activation products in the circulation and urine of primary membranous nephropathy.BMC Nephrol. 2019 Aug 9;20(1):313. doi: 10.1186/s12882-019-1509-5. BMC Nephrol. 2019. PMID: 31399080 Free PMC article.
-
Discordance of light chain isotypes between serum and glomerular deposits in proliferative glomerulonephritis with monoclonal IgG deposits: a case report and review of the literature.BMC Nephrol. 2023 Jul 1;24(1):199. doi: 10.1186/s12882-023-03256-5. BMC Nephrol. 2023. PMID: 37393252 Free PMC article. Review.
-
Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits complicated by immunoglobulin A nephropathy in the renal allograft.Nephrology (Carlton). 2016 Jul;21 Suppl 1:48-52. doi: 10.1111/nep.12775. Nephrology (Carlton). 2016. PMID: 26971743 Review.
Cited by
-
Daratumumab for Bortezomib-Refractory Proliferative Glomerulonephritis With Monoclonal Immunoglobulin Deposits in Kidney Allograft: A Case Report.Kidney Med. 2024 Dec 15;7(2):100945. doi: 10.1016/j.xkme.2024.100945. eCollection 2025 Feb. Kidney Med. 2024. PMID: 39895853 Free PMC article.
-
Complement abnormality predisposes to the development of malignant hypertension-associated thrombotic microangiopathy disease.Clin Kidney J. 2025 Jul 24;18(8):sfaf235. doi: 10.1093/ckj/sfaf235. eCollection 2025 Aug. Clin Kidney J. 2025. PMID: 40861385 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

