Mitochondria-derived Vesicle Packaging as a Novel Therapeutic Mechanism in Pulmonary Hypertension
- PMID: 37713305
- PMCID: PMC10768832
- DOI: 10.1165/rcmb.2023-0010OC
Mitochondria-derived Vesicle Packaging as a Novel Therapeutic Mechanism in Pulmonary Hypertension
Abstract
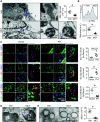
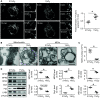
Increasing evidence suggests that mitochondrial dysfunction in pulmonary endothelial cells (ECs) plays a causative role in the initiation and progression of pulmonary hypertension (PH); how mitochondria become dysfunctional in PH remains elusive. Mitochondria-derived vesicles (MDVs) are small subcellular vesicles that excise from mitochondria. Whether MDV deregulation causes mitochondrial dysfunction in PH is unknown. The aim of this study was to determine MDV regulation in ECs and to elucidate how MDV deregulation in ECs leads to PH. MDV formation and mitochondrial morphology/dynamics were examined in ECs of EC-specific liver kinase B1 (LKB1) knockout mice (LKB1ec-/-), in monocrotaline-induced PH rats, and in lungs of patients with PH. Pulmonary ECs of patients with PH and hypoxia-treated pulmonary ECs exhibited increased mitochondrial fragmentation and disorganized mitochondrial ultrastructure characterized by electron lucent-swelling matrix compartments and concentric layering of the cristae network, together with defective MDV shedding. MDVs actively regulated mitochondrial membrane dynamics and mitochondrial ultrastructure via removing mitofission-related cargoes. The shedding of MDVs from parental mitochondria required LKB1-mediated mitochondrial recruitment of Rab9 GTPase. LKB1ec-/- mice spontaneously developed PH with decreased mitochondrial pools of Rab9 GTPase, defective MDV shedding, and disequilibrium of the mitochondrial fusion-fission cycle in pulmonary ECs. Aerosol intratracheal delivery of adeno-associated virus LKB1 reversed PH, together with improved MDV shedding and mitochondrial function in rats in vivo. We conclude that LKB1 regulates MDV shedding and mitochondrial dynamics in pulmonary ECs by enhancing mitochondrial recruitment of Rab9 GTPase. Defects of LKB1-mediated MDV shedding from parental mitochondria instigate EC dysfunction and PH.
Keywords: endothelial cell dysfunction; liver kinase B1; mitochondria-derived vesicles; pulmonary hypertension.
Figures



Comment in
-
LKB1 Regulates Pulmonary Hypertension Endothelial Cell Mitochondria: Another Layer of the Pulmonary Vascular Onion?Am J Respir Cell Mol Biol. 2024 Jan;70(1):5-7. doi: 10.1165/rcmb.2023-0322ED. Am J Respir Cell Mol Biol. 2024. PMID: 37738621 Free PMC article. No abstract available.
Similar articles
-
Flk1 Deficiency and Hypoxia Synergistically Promote Endothelial Dysfunction, Vascular Remodeling, and Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2023 Sep;43(9):1668-1683. doi: 10.1161/ATVBAHA.123.319266. Epub 2023 Aug 3. Arterioscler Thromb Vasc Biol. 2023. PMID: 37534464
-
Endothelial FIS1 DeSUMOylation Protects Against Hypoxic Pulmonary Hypertension.Circ Res. 2023 Sep;133(6):508-531. doi: 10.1161/CIRCRESAHA.122.321200. Epub 2023 Aug 17. Circ Res. 2023. PMID: 37589160
-
Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system.J Physiol. 2016 Sep 15;594(18):5343-62. doi: 10.1113/JP272703. Epub 2016 Jul 24. J Physiol. 2016. PMID: 27311616 Free PMC article.
-
Mitochondrial-Derived Vesicles-Link to Extracellular Vesicles and Implications in Cardiovascular Disease.Int J Mol Sci. 2023 Jan 30;24(3):2637. doi: 10.3390/ijms24032637. Int J Mol Sci. 2023. PMID: 36768960 Free PMC article. Review.
-
Mitochondria break free: Mitochondria-derived vesicles in aging and associated conditions.Ageing Res Rev. 2024 Dec;102:102549. doi: 10.1016/j.arr.2024.102549. Epub 2024 Oct 19. Ageing Res Rev. 2024. PMID: 39427885 Review.
Cited by
-
LKB1 Regulates Pulmonary Hypertension Endothelial Cell Mitochondria: Another Layer of the Pulmonary Vascular Onion?Am J Respir Cell Mol Biol. 2024 Jan;70(1):5-7. doi: 10.1165/rcmb.2023-0322ED. Am J Respir Cell Mol Biol. 2024. PMID: 37738621 Free PMC article. No abstract available.
-
Mitochondria-derived vesicles: A promising and potential target for tumour therapy.Clin Transl Med. 2025 May;15(5):e70320. doi: 10.1002/ctm2.70320. Clin Transl Med. 2025. PMID: 40356246 Free PMC article. Review.
-
Liver Kinase B1 Protects Against Hypoxia-Induced Pulmonary Arterial Endothelial Cell Dysfunction via the AMP-Activated Protein Kinase Pathway.Biochem Genet. 2024 Dec 25. doi: 10.1007/s10528-024-11007-w. Online ahead of print. Biochem Genet. 2024. PMID: 39720983
References
-
- Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, et al. Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem . 2004;279:37575–37587. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

