Nanomaterial genotoxicity evaluation using the high-throughput p53-binding protein 1 (53BP1) assay
- PMID: 37713377
- PMCID: PMC10503773
- DOI: 10.1371/journal.pone.0288737
Nanomaterial genotoxicity evaluation using the high-throughput p53-binding protein 1 (53BP1) assay
Abstract
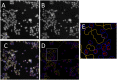
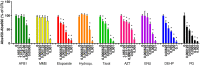
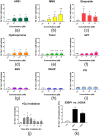
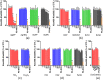
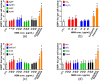
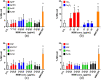
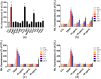
Toxicity evaluation of engineered nanomaterials is challenging due to the ever increasing number of materials and because nanomaterials (NMs) frequently interfere with commonly used assays. Hence, there is a need for robust, high-throughput assays with which to assess their hazard potential. The present study aimed at evaluating the applicability of a genotoxicity assay based on the immunostaining and foci counting of the DNA repair protein 53BP1 (p53-binding protein 1), in a high-throughput format, for NM genotoxicity assessment. For benchmarking purposes, we first applied the assay to a set of eight known genotoxic agents, as well as X-ray irradiation (1 Gy). Then, a panel of NMs and nanobiomaterials (NBMs) was evaluated with respect to their impact on cell viability and genotoxicity, and to their potential to induce reactive oxygen species (ROS) production. The genotoxicity recorded using the 53BP1 assay was confirmed using the micronucleus assay, also scored via automated (high-throughput) microscopy. The 53BP1 assay successfully identified genotoxic compounds on the HCT116 human intestinal cell line. None of the tested NMs showed any genotoxicity using the 53BP1 assay, except the positive control consisting in (CoO)(NiO) NMs, while only TiO2 NMs showed positive outcome in the micronucleus assay. Only Fe3O4 NMs caused significant elevation of ROS, not correlated to DNA damage. Therefore, owing to its adequate predictivity of the genotoxicity of most of the tested benchmark substance and its ease of implementation in a high throughput format, the 53BP1 assay could be proposed as a complementary high-throughput screening genotoxicity assay, in the context of the development of New Approach Methodologies.
Copyright: © 2023 Fontaine et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Hazard Assessment of Benchmark Metal-Based Nanomaterials Through a Set of In Vitro Genotoxicity Assays.Adv Exp Med Biol. 2022;1357:351-375. doi: 10.1007/978-3-030-88071-2_14. Adv Exp Med Biol. 2022. PMID: 35583651
-
New methodological developments for testing the in vitro genotoxicity of nanomaterials: Comparison of 2D and 3D HepaRG liver cell models and classical and high throughput comet assay formats.Chemosphere. 2024 Feb;350:140975. doi: 10.1016/j.chemosphere.2023.140975. Epub 2023 Dec 22. Chemosphere. 2024. PMID: 38142884
-
An in vitro assessment of panel of engineered nanomaterials using a human renal cell line: cytotoxicity, pro-inflammatory response, oxidative stress and genotoxicity.BMC Nephrol. 2013 Apr 25;14:96. doi: 10.1186/1471-2369-14-96. BMC Nephrol. 2013. PMID: 23617532 Free PMC article.
-
Emerging metrology for high-throughput nanomaterial genotoxicology.Mutagenesis. 2017 Jan;32(1):215-232. doi: 10.1093/mutage/gew037. Epub 2016 Aug 26. Mutagenesis. 2017. PMID: 27565834 Free PMC article. Review.
-
Genotoxicity Assessment of Nanomaterials: Recommendations on Best Practices, Assays, and Methods.Toxicol Sci. 2018 Aug 1;164(2):391-416. doi: 10.1093/toxsci/kfy100. Toxicol Sci. 2018. PMID: 29701824 Review.
Cited by
-
Iron Oxide (Magnetite)-Based Nanobiomaterial with Medical Applications-Environmental Hazard Assessment Using Terrestrial Model Species.J Xenobiot. 2024 Feb 22;14(1):285-294. doi: 10.3390/jox14010017. J Xenobiot. 2024. PMID: 38535492 Free PMC article.
-
On the lifespan of Enchytraeus crypticus - impact of iron (nanomaterial and salt) on aging.Aging (Albany NY). 2024 Oct 24;16(20):13012-13024. doi: 10.18632/aging.206134. Epub 2024 Oct 24. Aging (Albany NY). 2024. PMID: 39448087 Free PMC article.
References
-
- MacCormack TJ, Meli MV, Ede JD, Ong KJ, Rourke JL, Dieni CA. Commentary: Revisiting nanoparticle-assay interference: There’s plenty of room at the bottom for misinterpretation. Comparative biochemistry and physiology Part B, Biochemistry & molecular biology. 2021;255:110601. doi: 10.1016/j.cbpb.2021.110601 - DOI - PubMed
-
- Guadagnini R, Halamoda Kenzaoui B, Walker L, Pojana G, Magdolenova Z, Bilanicova D, et al.. Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology. 2015;9 Suppl 1:13–24. doi: 10.3109/17435390.2013.829590 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

