The image-based ultrasonic cell shaking test
- PMID: 37713387
- PMCID: PMC10503762
- DOI: 10.1371/journal.pone.0285906
The image-based ultrasonic cell shaking test
Abstract
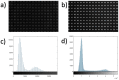
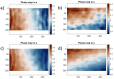
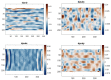
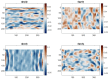
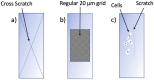
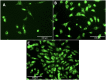
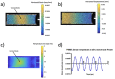
Mechanical signals play a vital role in cell biology and is a vast area of research. Thus, there is motivation to understand cell deformation and mechanobiological responses. However, the ability to controllably deform cells in the ultrasonic regime and test their response is a noted challenge throughout the literature. Quantifying and eliciting an appropriate stimulus has proven to be difficult, resulting in methods that are either too aggressive or oversimplified. Furthermore, the ability to gain a real-time insight into cell deformation and link this with the biological response is yet to be achieved. One application of this understanding is in ultrasonic surgical cutting, which is a promising alternative to traditional methods, but with little understanding of its effect on cells. Here we present the image based ultrasonic cell shaking test, a novel method that enables controllable loading of cells and quantification of their response to ultrasonic vibrations. Practically, this involves seeding cells on a substrate that resonates at ultrasonic frequencies and transfers the deformation to the cells. This is then incorporated into microscopic imaging techniques to obtain high-speed images of ultrasonic cell deformation that can be analysed using digital image correlation techniques. Cells can then be extracted after excitation to undergo analysis to understand the biological response to the deformation. This method could aid in understanding the effects of ultrasonic stimulation on cells and how activated mechanobiological pathways result in physical and biochemical responses.
Copyright: © 2023 Ballard et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






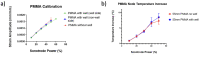

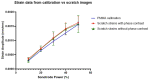
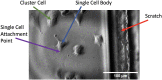
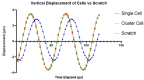

References
-
- O’Daly BJ, Morris E, Gavin GP, O’Byrne JM, McGuinness GB, High-power low-frequency ultrasound: A review of tissue dissection and ablation in medicine and surgery Journal of Materials Processing Technology. 2008. May;200:38–58. doi: 10.1016/j.jmatprotec.2007.11.041 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

