Levels and in vitro functional effects of circulating anti-hinge antibodies in melanoma patients receiving the immune checkpoint inhibitor pembrolizumab
- PMID: 37713423
- PMCID: PMC10503750
- DOI: 10.1371/journal.pone.0290793
Levels and in vitro functional effects of circulating anti-hinge antibodies in melanoma patients receiving the immune checkpoint inhibitor pembrolizumab
Abstract
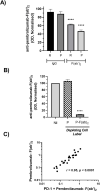
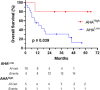
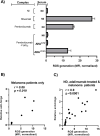
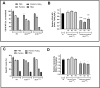
The efficacy of PD-1 monoclonals such as pembrolizumab can be modulated by the signals delivered via their Fc region. Tumour/inflammation associated proteases can generate F(ab')2 fragments of therapeutic monoclonals, and subsequent recognition of F(ab')2 epitopes by circulating anti-hinge antibodies (AHA) can then, potentially, link F(ab')2 binding to the target antigen with novel Fc signalling. Although elevated in inflammatory diseases, AHA levels in cancer patients have not been investigated and functional studies utilising the full repertoire of AHA present in sera have been limited. AHA levels in pembrolizumab treated melanoma patients (n = 23) were therefore compared to those of normal donors and adalimumab treated patients. A subset of melanoma patients and the majority of adalimumab patients had elevated levels of AHA reactive with F(ab')2 fragments of IgG4 anti-PD-1 monoclonals (nivolumab, pembrolizumab) and IgG1 therapeutic monoclonals (rituximab, adalimumab). Survival analysis was restricted by the small patient numbers but those melanoma patients with the highest levels (>75% percentile, n = 5) of pembrolizumab-F(ab')2 reactive AHA had significantly better overall survival post pembrolizumab treatment (p = 0.039). In vitro functional studies demonstrated that the presence of AHA+ sera restored the neutrophil activating capacity of pembrolizumab to its F(ab')2 fragment. Neither pembrolizumab nor its F(ab')2 fragments can induce NK cell or complement dependent cytotoxicity (CDC). However, AHA+ sera in combination with pembrolizumab-F(ab')2 provided Fc regions that could activate NK cells. The ability of AHA+ sera to restore CDC activity was more restricted and observed using only one pembrolizumab and one adalimumab patient serum in combination with rituximab- F(ab')2. This study reports the presence of elevated AHA levels in pembrolizumab treated melanoma patients and highlight the potential for AHA to provide additional Fc signaling. The issue of whether tumour associated proteolysis of PD-1 mAbs and subsequent AHA recognition impacts on treatment efficacy requires further study.
Copyright: © 2023 Hock et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures

Similar articles
-
IgG anti-hinge antibodies against IgG4 F(ab')2 fragments generated using pepsin are useful diagnostic markers for rheumatoid arthritis: implications of the possible roles of metalloproteinases and IgG subclasses in generating immunogenic hinge epitopes.Arthritis Res Ther. 2020 Jun 26;22(1):161. doi: 10.1186/s13075-020-02251-7. Arthritis Res Ther. 2020. PMID: 32586370 Free PMC article.
-
A monoclonal antibody against hinge-cleaved IgG restores effector function to proteolytically-inactivated IgGs in vitro and in vivo.MAbs. 2014;6(5):1265-73. doi: 10.4161/mabs.29825. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517311 Free PMC article.
-
Anti-Hinge Antibodies Recognize IgG Subclass- and Protease-Restricted Neoepitopes.J Immunol. 2017 Jan 1;198(1):82-93. doi: 10.4049/jimmunol.1601096. Epub 2016 Nov 18. J Immunol. 2017. PMID: 27864476
-
Stimulation of complement amplification by F(ab')(2)-containing immune complexes and naturally occurring anti-hinge antibodies, possible role in systemic inflammation.Autoimmun Rev. 2008 Jun;7(6):508-13. doi: 10.1016/j.autrev.2008.04.017. Epub 2008 May 12. Autoimmun Rev. 2008. PMID: 18558371 Review.
-
How immune complexes from certain IgG NAbs and any F(ab')₂ can mediate excessive complement activation.Adv Exp Med Biol. 2012;750:186-96. doi: 10.1007/978-1-4614-3461-0_14. Adv Exp Med Biol. 2012. PMID: 22903675 Free PMC article. Review.
Cited by
-
Impact of rheumatoid factors on the function of therapeutic monoclonals specific for PD-1/PD-L1.Cancer Immunol Immunother. 2025 May 24;74(7):216. doi: 10.1007/s00262-025-04078-0. Cancer Immunol Immunother. 2025. PMID: 40411581 Free PMC article.
References
-
- Lo Russo G, Moro M, Sommariva M, Cancila V, Boeri M, Centonze G, et al.. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res. 2019;25(3):989–99. doi: 10.1158/1078-0432.CCR-18-1390 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

