A new polymodal gating model of the proton-activated chloride channel
- PMID: 37713449
- PMCID: PMC10529583
- DOI: 10.1371/journal.pbio.3002309
A new polymodal gating model of the proton-activated chloride channel
Abstract
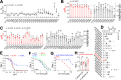
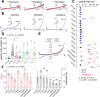
The proton-activated chloride (PAC) channel plays critical roles in ischemic neuron death, but its activation mechanisms remain elusive. Here, we investigated the gating of PAC channels using its novel bifunctional modulator C77304. C77304 acted as a weak activator of the PAC channel, causing moderate activation by acting on its proton gating. However, at higher concentrations, C77304 acted as a weak inhibitor, suppressing channel activity. This dual function was achieved by interacting with 2 modulatory sites of the channel, each with different affinities and dependencies on the channel's state. Moreover, we discovered a protonation-independent voltage activation of the PAC channel that appears to operate through an ion-flux gating mechanism. Through scanning-mutagenesis and molecular dynamics simulation, we confirmed that E181, E257, and E261 in the human PAC channel serve as primary proton sensors, as their alanine mutations eliminated the channel's proton gating while sparing the voltage-dependent gating. This proton-sensing mechanism was conserved among orthologous PAC channels from different species. Collectively, our data unveils the polymodal gating and proton-sensing mechanisms in the PAC channel that may inspire potential drug development.
Copyright: © 2023 Zhao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Molecular determinants of pH sensing in the proton-activated chloride channel.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2200727119. doi: 10.1073/pnas.2200727119. Epub 2022 Jul 25. Proc Natl Acad Sci U S A. 2022. PMID: 35878032 Free PMC article.
-
Identification of the Acid-Sensitive Site Critical for Chloral Hydrate (CH) Activation of the Proton-Activated Chloride Channel.J Neurosci. 2023 Jan 25;43(4):526-539. doi: 10.1523/JNEUROSCI.0482-22.2022. Epub 2022 Oct 25. J Neurosci. 2023. PMID: 36283831 Free PMC article.
-
An S6 mutation in BK channels reveals beta1 subunit effects on intrinsic and voltage-dependent gating.J Gen Physiol. 2006 Dec;128(6):731-44. doi: 10.1085/jgp.200609596. J Gen Physiol. 2006. PMID: 17130522 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Temperature-sensitive gating of voltage-gated proton channels.Curr Top Membr. 2014;74:259-92. doi: 10.1016/B978-0-12-800181-3.00010-5. Curr Top Membr. 2014. PMID: 25366240 Review.
Cited by
-
Preferential allosteric modulation of Otop1 channels by small molecule compounds.Commun Biol. 2025 Feb 26;8(1):314. doi: 10.1038/s42003-025-07775-9. Commun Biol. 2025. PMID: 40011703 Free PMC article.
References
LinkOut - more resources
Full Text Sources

