Sodium channel endocytosis drives axon initial segment plasticity
- PMID: 37713493
- PMCID: PMC10881073
- DOI: 10.1126/sciadv.adf3885
Sodium channel endocytosis drives axon initial segment plasticity
Abstract
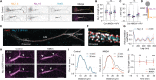
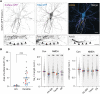
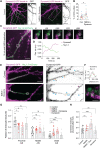
Activity-dependent plasticity of the axon initial segment (AIS) endows neurons with the ability to adapt action potential output to changes in network activity. Action potential initiation at the AIS highly depends on the clustering of voltage-gated sodium channels, but the molecular mechanisms regulating their plasticity remain largely unknown. Here, we developed genetic tools to label endogenous sodium channels and their scaffolding protein, to reveal their nanoscale organization and longitudinally image AIS plasticity in hippocampal neurons in slices and primary cultures. We find that N-methyl-d-aspartate receptor activation causes both long-term synaptic depression and rapid internalization of AIS sodium channels within minutes. The clathrin-mediated endocytosis of sodium channels at the distal AIS increases the threshold for action potential generation. These data reveal a fundamental mechanism for rapid activity-dependent AIS reorganization and suggests that plasticity of intrinsic excitability shares conserved features with synaptic plasticity.
Figures



References
-
- M. H. Kole, G. J. Stuart, Signal processing in the axon initial segment. Neuron 73, 235–247 (2012). - PubMed
-
- J. J. Garrido, P. Giraud, E. Carlier, F. Fernandes, A. Moussif, M. P. Fache, D. Debanne, B. Dargent, A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300, 2091–2094 (2003). - PubMed
-
- G. Lemaillet, B. Walker, S. Lambert, Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J. Biol. Chem. 278, 27333–27339 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

