TRAPPC6B biallelic variants cause a neurodevelopmental disorder with TRAPP II and trafficking disruptions
- PMID: 37713627
- PMCID: PMC10766242
- DOI: 10.1093/brain/awad301
TRAPPC6B biallelic variants cause a neurodevelopmental disorder with TRAPP II and trafficking disruptions
Abstract
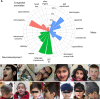
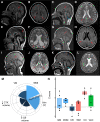
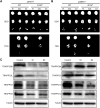
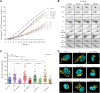
Highly conserved transport protein particle (TRAPP) complexes regulate subcellular trafficking pathways. Accurate protein trafficking has been increasingly recognized to be critically important for normal development, particularly in the nervous system. Variants in most TRAPP complex subunits have been found to lead to neurodevelopmental disorders with diverse but overlapping phenotypes. We expand on limited prior reports on TRAPPC6B with detailed clinical and neuroradiologic assessments, and studies on mechanisms of disease, and new types of variants. We describe 29 additional patients from 18 independent families with biallelic variants in TRAPPC6B. We identified seven homozygous nonsense (n = 12 patients) and eight canonical splice-site variants (n = 17 patients). In addition, we identified one patient with compound heterozygous splice-site/missense variants with a milder phenotype and one patient with homozygous missense variants. Patients displayed non-progressive microcephaly, global developmental delay/intellectual disability, epilepsy and absent expressive language. Movement disorders including stereotypies, spasticity and dystonia were also observed. Brain imaging revealed reductions in cortex, cerebellum and corpus callosum size with frequent white matter hyperintensity. Volumetric measurements indicated globally diminished volume rather than specific regional losses. We identified a reduced rate of trafficking into the Golgi apparatus and Golgi fragmentation in patient-derived fibroblasts that was rescued by wild-type TRAPPC6B. Molecular studies revealed a weakened interaction between mutant TRAPPC6B (c.454C>T, p.Q152*) and its TRAPP binding partner TRAPPC3. Patient-derived fibroblasts from the TRAPPC6B (c.454C>T, p.Q152*) variant displayed reduced levels of TRAPPC6B as well as other TRAPP II complex-specific members (TRAPPC9 and TRAPPC10). Interestingly, the levels of the TRAPPC6B homologue TRAPPC6A were found to be elevated. Moreover, co-immunoprecipitation experiments showed that TRAPPC6A co-precipitates equally with TRAPP II and TRAPP III, while TRAPPC6B co-precipitates significantly more with TRAPP II, suggesting enrichment of the protein in the TRAPP II complex. This implies that variants in TRAPPC6B may preferentially affect TRAPP II functions compared to TRAPP III functions. Finally, we assessed phenotypes in a Drosophila TRAPPC6B-deficiency model. Neuronal TRAPPC6B knockdown impaired locomotion and led to wing posture defects, supporting a role for TRAPPC6B in neuromotor function. Our findings confirm the association of damaging biallelic TRAPPC6B variants with microcephaly, intellectual disability, language impairments, and epilepsy. A subset of patients also exhibited dystonia and/or spasticity with impaired ambulation. These features overlap with disorders arising from pathogenic variants in other TRAPP subunits, particularly components of the TRAPP II complex. These findings suggest that TRAPPC6B is essential for brain development and function, and TRAPP II complex activity may be particularly relevant for mediating this function.
Keywords: ER-golgi trafficking; NEDMEBA; TRAPP-II complex; TRAPPC6B; TRAPPopathy; Trs33.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

