Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death
- PMID: 37713808
- PMCID: PMC10507138
- DOI: 10.1016/j.ebiom.2023.104784
Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death
Abstract
Background: We have recently demonstrated a causal link between loss of gonadotropin-releasing hormone (GnRH), the master molecule regulating reproduction, and cognitive deficits during pathological aging, including Down syndrome and Alzheimer's disease. Olfactory and cognitive alterations, which persist in some COVID-19 patients, and long-term hypotestosteronaemia in SARS-CoV-2-infected men are also reminiscent of the consequences of deficient GnRH, suggesting that GnRH system neuroinvasion could underlie certain post-COVID symptoms and thus lead to accelerated or exacerbated cognitive decline.
Methods: We explored the hormonal profile of COVID-19 patients and targets of SARS-CoV-2 infection in post-mortem patient brains and human fetal tissue.
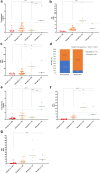
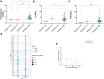
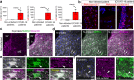
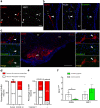
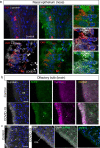
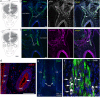
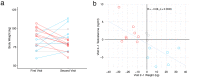
Findings: We found that persistent hypotestosteronaemia in some men could indeed be of hypothalamic origin, favouring post-COVID cognitive or neurological symptoms, and that changes in testosterone levels and body weight over time were inversely correlated. Infection of olfactory sensory neurons and multifunctional hypothalamic glia called tanycytes highlighted at least two viable neuroinvasion routes. Furthermore, GnRH neurons themselves were dying in all patient brains studied, dramatically reducing GnRH expression. Human fetal olfactory and vomeronasal epithelia, from which GnRH neurons arise, and fetal GnRH neurons also appeared susceptible to infection.
Interpretation: Putative GnRH neuron and tanycyte dysfunction following SARS-CoV-2 neuroinvasion could be responsible for serious reproductive, metabolic, and mental health consequences in long-COVID and lead to an increased risk of neurodevelopmental and neurodegenerative pathologies over time in all age groups.
Funding: European Research Council (ERC) grant agreements No 810331, No 725149, No 804236, the European Union Horizon 2020 research and innovation program No 847941, the Fondation pour la Recherche Médicale (FRM) and the Agence Nationale de la Recherche en Santé (ANRS) No ECTZ200878 Long Covid 2021 ANRS0167 SIGNAL, Agence Nationale de la recherche (ANR) grant agreements No ANR-19-CE16-0021-02, No ANR-11-LABEX-0009, No. ANR-10-LABEX-0046, No. ANR-16-IDEX-0004, Inserm Cross-Cutting Scientific Program HuDeCA, the CHU Lille Bonus H, the UK Medical Research Council (MRC) and National Institute of Health and care Research (NIHR).
Keywords: COVID-19; Cognition; GnRH; Hypothalamus; Infertility; Neurodevelopment.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interest.
Figures


References
-
- Dierssen M. Down syndrome: the brain in trisomic mode. Nat Rev Neurosci. 2012;13(12):844–858. - PubMed
-
- Casoni F., Malone S.A., Belle M., et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969–3981. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

