Deficiency in AK9 causes asthenozoospermia and male infertility by destabilising sperm nucleotide homeostasis
- PMID: 37713809
- PMCID: PMC10507140
- DOI: 10.1016/j.ebiom.2023.104798
Deficiency in AK9 causes asthenozoospermia and male infertility by destabilising sperm nucleotide homeostasis
Erratum in
-
Corrigendum to "Deficiency in AK9 causes asthenozoospermia and male infertility by destabilising sperm nucleotide homeostasis" [EBioMedicine 96(2023) 104798].EBioMedicine. 2024 Jan;99:104957. doi: 10.1016/j.ebiom.2023.104957. Epub 2024 Jan 3. EBioMedicine. 2024. PMID: 38171079 Free PMC article. No abstract available.
Abstract
Background: Asthenozoospermia is the primary cause of male infertility; however, its genetic aetiology remains poorly understood. Adenylate kinase 9 (AK9) is highly expressed in the testes of humans and mice and encodes a type of adenosine kinase that is functionally involved in cellular nucleotide homeostasis and energy metabolism. We aimed to assess whether AK9 is involved in asthenozoospermia.
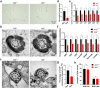
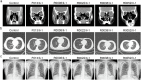
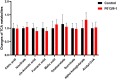
Methods: One-hundred-and-sixty-five Chinese men with idiopathic asthenozoospermia were recruited. Whole-exome sequencing (WES) and Sanger sequencing were performed for genetic analyses. Papanicolaou staining, Haematoxylin and eosin staining, scanning electron microscopy, and transmission electron microscopy were used to observe the sperm morphology and structure. Ak9-knockout mice were generated using CRISPR-Cas9. Sperm adenosine was detected by liquid chromatography-mass spectrometry. Targeted sperm metabolomics was performed. Intracytoplasmic sperm injection (ICSI) was used to treat patients.
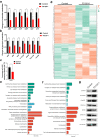
Findings: We identified five patients harbouring bi-allelic AK9 mutations. Spermatozoa from men harbouring bi-allelic AK9 mutations have a decreased ability to sustain nucleotide homeostasis. Moreover, bi-allelic AK9 mutations inhibit glycolysis in sperm. Ak9-knockout male mice also presented similar phenotypes of asthenozoospermia. Interestingly, ICSI was effective in bi-allelic AK9 mutant patients in achieving good pregnancy outcomes.
Interpretation: Defects in AK9 induce asthenozoospermia with defects in nucleotide homeostasis and energy metabolism. This sterile phenotype could be rescued by ICSI.
Funding: The National Natural Science Foundation of China (82071697), Medical Innovation Project of Fujian Province (2020-CXB-051), open project of the NHC Key Laboratory of Male Reproduction and Genetics in Guangzhou (KF202004), Medical Research Foundation of Guangdong Province (A2021269), Guangdong Provincial Reproductive Science Institute Innovation Team grants (C-03), and Outstanding Young Talents Program of Capital Medical University (B2205).
Keywords: AK9; Asthenozoospermia; Energetic metabolism; Intracytoplasmic sperm injection; Nucleotide homeostasis.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interest.
Figures








Similar articles
-
Biallelic mutations in ARMC12 cause asthenozoospermia and multiple midpiece defects in humans and mice.J Med Genet. 2023 Feb;60(2):154-162. doi: 10.1136/jmedgenet-2021-108137. Epub 2022 May 9. J Med Genet. 2023. PMID: 35534203
-
Genetic diagnosis, sperm phenotype and ICSI outcome in case of severe asthenozoospermia with multiple morphological abnormalities of the flagellum.Hum Reprod. 2021 Oct 18;36(11):2848-2860. doi: 10.1093/humrep/deab200. Hum Reprod. 2021. PMID: 34529793
-
Biallelic variants in KCTD19 associated with male factor infertility and oligoasthenoteratozoospermia.Hum Reprod. 2023 Jul 5;38(7):1399-1411. doi: 10.1093/humrep/dead095. Hum Reprod. 2023. PMID: 37192818
-
Genetic underpinnings of asthenozoospermia.Best Pract Res Clin Endocrinol Metab. 2020 Dec;34(6):101472. doi: 10.1016/j.beem.2020.101472. Epub 2020 Nov 6. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 33191078 Review.
-
Metabolic Dysregulation and Sperm Motility in Male Infertility.Adv Exp Med Biol. 2022;1358:257-273. doi: 10.1007/978-3-030-89340-8_12. Adv Exp Med Biol. 2022. PMID: 35641874 Review.
Cited by
-
Baicalin targets YTHDC2 and alleviates male reproductive toxicity caused by co-exposure to nanoplastics and manganese through m6A-dependent pathway.J Nanobiotechnology. 2025 Jun 18;23(1):450. doi: 10.1186/s12951-025-03535-3. J Nanobiotechnology. 2025. PMID: 40533738 Free PMC article.
-
Biallelic loss-of-function variants of DNAH7 cause male infertility associated with asthenozoospermia in humans.Hum Genet. 2025 Aug 14. doi: 10.1007/s00439-025-02766-6. Online ahead of print. Hum Genet. 2025. PMID: 40810911
-
Mouse radial spoke 3 is a metabolic and regulatory hub in cilia.Nat Struct Mol Biol. 2025 Aug;32(8):1542-1554. doi: 10.1038/s41594-025-01594-6. Epub 2025 Jun 27. Nat Struct Mol Biol. 2025. PMID: 40579595
-
Human asthenozoospermia: Update on genetic causes, patient management, and clinical strategies.Andrology. 2025 Jul;13(5):1044-1064. doi: 10.1111/andr.13828. Epub 2025 Jan 2. Andrology. 2025. PMID: 39748639 Free PMC article. Review.
-
Acupuncture mediates the "gut-testis axis" to improve asthenozoospermia.Front Endocrinol (Lausanne). 2025 Jan 27;16:1514010. doi: 10.3389/fendo.2025.1514010. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 39959619 Free PMC article.
References
-
- Vander Borght M., Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. - PubMed
-
- Dohle G.R., Colpi G.M., Hargreave T.B., et al. EAU guidelines on male infertility. Eur Urol. 2005;48(5):703–711. - PubMed
-
- Jiao S.Y., Yang Y.H., Chen S.R. Molecular genetics of infertility: loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum Reprod Update. 2021;27(1):154–189. - PubMed
-
- Chemes H.E. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21(6):799–808. - PubMed
-
- Curi S.M., Ariagno J.I., Chenlo P.H., et al. Asthenozoospermia: analysis of a large population. Arch Androl. 2003;49(5):343–349. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

