Role of Translesion DNA Synthesis in the Metabolism of Replication-associated Nascent Strand Gaps
- PMID: 37714300
- PMCID: PMC10842951
- DOI: 10.1016/j.jmb.2023.168275
Role of Translesion DNA Synthesis in the Metabolism of Replication-associated Nascent Strand Gaps
Abstract
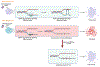
Translesion DNA synthesis (TLS) is a DNA damage tolerance pathway utilized by cells to overcome lesions encountered throughout DNA replication. During replication stress, cancer cells show increased dependency on TLS proteins for cellular survival and chemoresistance. TLS proteins have been described to be involved in various DNA repair pathways. One of the major emerging roles of TLS is single-stranded DNA (ssDNA) gap-filling, primarily after the repriming activity of PrimPol upon encountering a lesion. Conversely, suppression of ssDNA gap accumulation by TLS is considered to represent a mechanism for cancer cells to evade the toxicity of chemotherapeutic agents, specifically in BRCA-deficient cells. Thus, TLS inhibition is emerging as a potential treatment regimen for DNA repair-deficient tumors.
Keywords: BRCA; homologous recombination; replication stress; ssDNA gaps; translesion synthesis.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

