A cardiac amino-terminal GRK2 peptide inhibits insulin resistance yet enhances maladaptive cardiovascular and brown adipose tissue remodeling in females during diet-induced obesity
- PMID: 37714510
- PMCID: PMC10591815
- DOI: 10.1016/j.yjmcc.2023.09.001
A cardiac amino-terminal GRK2 peptide inhibits insulin resistance yet enhances maladaptive cardiovascular and brown adipose tissue remodeling in females during diet-induced obesity
Abstract
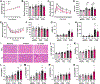
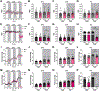
Obesity and metabolic disorders are increasing in epidemic proportions, leading to poor outcomes including heart failure. With a growing recognition of the effect of adipose tissue dysfunction on heart disease, it is less well understood how the heart can influence systemic metabolic homeostasis. Even less well understood is sex differences in cardiometabolic responses. Previously, our lab investigated the role of the amino-terminus of GRK2 in cardiometabolic remodeling using transgenic mice with cardiac restricted expression of a short peptide, βARKnt. Male mice preserved insulin sensitivity, enhanced metabolic flexibility and adipose tissue health, elicited cardioprotection, and improved cardiac metabolic signaling. To examine the effect of cardiac βARKnt expression on cardiac and metabolic function in females in response to diet-induced obesity, we subjected female mice to high fat diet (HFD) to trigger cardiac and metabolic adaptive changes. Despite equivalent weight gain, βARKnt mice exhibited improved glucose tolerance and insulin sensitivity. However, βARKnt mice displayed a progressive reduction in energy expenditure during cold challenge after acute and chronic HFD stress. They also demonstrated reduced cardiac function and increased markers of maladaptive remodeling and tissue injury, and decreased or aberrant metabolic signaling. βARKnt mice exhibited reduced lipid deposition in the brown adipose tissue (BAT), but delayed or decreased markers of BAT activation and function suggested multiple mechanisms contributed to the decreased thermogenic capacity. These data suggest a non-canonical cardiac regulation of BAT lipolysis and function that highlights the need for studies elucidating the mechanisms of sex-specific responses to metabolic dysfunction.
Keywords: Lipolysis; Metabolism; Obesity; Thermogenesis.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures
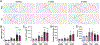
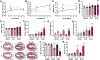
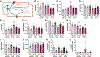
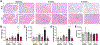
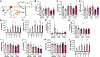

Similar articles
-
Augmented CCL5/CCR5 signaling in brown adipose tissue inhibits adaptive thermogenesis and worsens insulin resistance in obesity.Clin Sci (Lond). 2022 Jan 14;136(1):121-137. doi: 10.1042/CS20210959. Clin Sci (Lond). 2022. PMID: 34821367
-
Time-restricted feeding improves metabolic syndrome by activating thermogenesis in brown adipose tissue and reducing inflammatory markers.Front Immunol. 2025 Jan 24;16:1501850. doi: 10.3389/fimmu.2025.1501850. eCollection 2025. Front Immunol. 2025. PMID: 39925816 Free PMC article.
-
Isoliquiritigenin Ameliorates High-Fat Diet-Induced Obesity in Mice by Activating Brown Adipose Tissue.Int J Mol Sci. 2025 Feb 14;26(4):1616. doi: 10.3390/ijms26041616. Int J Mol Sci. 2025. PMID: 40004080 Free PMC article.
-
Beyond obesity - thermogenic adipocytes and cardiometabolic health.Horm Mol Biol Clin Investig. 2017 May 26;31(2):/j/hmbci.2017.31.issue-2/hmbci-2017-0007/hmbci-2017-0007.xml. doi: 10.1515/hmbci-2017-0007. Horm Mol Biol Clin Investig. 2017. PMID: 28593918 Review.
-
Hypothalamic Estrogen Signaling and Adipose Tissue Metabolism in Energy Homeostasis.Front Endocrinol (Lausanne). 2022 Jun 9;13:898139. doi: 10.3389/fendo.2022.898139. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35757435 Free PMC article. Review.
References
-
- Koliaki C, Liatis S, Kokkinos A, Obesity and cardiovascular disease: revisiting an old relationship, Metabolism 92 (2019) 98–107. - PubMed
-
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J, Ziaeian B, 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, J Am Coll Cardiol 74(10) (2019) e177–e232. - PMC - PubMed
-
- Lavie CJ, McAuley PA, Church TS, Milani RV, Blair SN, Obesity and cardiovascular diseases: implications regarding fitness, fatness, and severity in the obesity paradox, J Am Coll Cardiol 63(14) (2014) 1345–54. - PubMed
-
- Lavie CJ, De Schutter A, Milani RV, Healthy obese versus unhealthy lean: the obesity paradox, Nat Rev Endocrinol 11(1) (2015) 55–62. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

