StereoPHIP: Stereoselective Parahydrogen-Induced Polarization
- PMID: 37714818
- PMCID: PMC10842948
- DOI: 10.1002/anie.202311669
StereoPHIP: Stereoselective Parahydrogen-Induced Polarization
Abstract
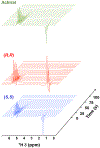
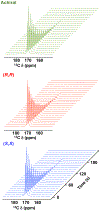
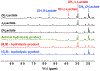
Parahydrogen-induced polarization (PHIP) followed by polarization transfer to 13 C is a rapidly developing technique for the generation of 13 C-hyperpolarized substrates. Chirality plays an essential role in living systems and differential metabolism of enantiomeric pairs of metabolic substrates is well documented. Inspired by asymmetric hydrogenation, here we report stereoPHIP, which involves the addition of parahydrogen to a prochiral substrate with a chiral catalyst followed by polarization transfer to 13 C spins. We demonstrate that parahydrogen could be rapidly added to the prochiral precursor to both enantiomers of lactic acid (D and L), with both the (R,R) and (S,S) enantiomers of a chiral rhodium(I) catalyst to afford highly 13 C-hyperpolarized (over 20 %) L- and D-lactate ester derivatives, respectively, with excellent stereoselectivity. We also show that the hyperpolarized 1 H signal decays obtained with the (R,R) and (S,S) catalysts were markedly different. StereoPHIP expands the scope of conventional PHIP to the production of 13 C hyperpolarized chiral substrates with high stereoselectivity.
Keywords: Lactate; NMR Spectroscopy; Parahydrogen-Induced Polarization; Pyruvate; Stereoselectivity.
© 2023 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Figures



References
-
- Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL, J. Am. Chem. Soc 1987, 109, 8089–8091.
-
- Reineri F, Cavallari E, Carrera C, Aime S, Magn. Reson. Mater. Phys. Biol. Med 2021, 34, 25–47.
-
- Reineri F, Anal. Sens 2022, 2, e202200028.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

