Selective effects of estradiol on human corneal endothelial cells
- PMID: 37714879
- PMCID: PMC10504266
- DOI: 10.1038/s41598-023-42290-z
Selective effects of estradiol on human corneal endothelial cells
Abstract
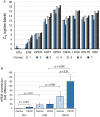
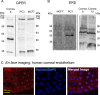
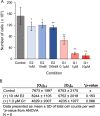
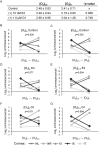
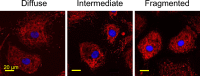
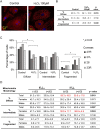
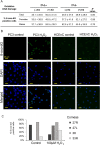
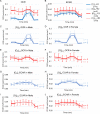
In Fuchs endothelial corneal dystrophy (FECD), mitochondrial and oxidative stresses in corneal endothelial cells (HCEnCs) contribute to cell demise and disease progression. FECD is more common in women than men, but the basis for this observation is poorly understood. To understand the sex disparity in FECD prevalence, we studied the effects of the sex hormone 17-β estradiol (E2) on growth, oxidative stress, and metabolism in primary cultures of HCEnCs grown under physiologic ([O2]2.5) and hyperoxic ([O2]A) conditions. We hypothesized that E2 would counter the damage of oxidative stress generated at [O2]A. HCEnCs were treated with or without E2 (10 nM) for 7-10 days under both conditions. Treatment with E2 did not significantly alter HCEnC density, viability, ROS levels, oxidative DNA damage, oxygen consumption rates, or extracellular acidification rates in either condition. E2 disrupted mitochondrial morphology in HCEnCs solely from female donors in the [O2]A condition. ATP levels were significantly higher at [O2]2.5 than at [O2]A in HCEnCs from female donors only, but were not affected by E2. Our findings demonstrate the resilience of HCEnCs against hyperoxic stress. The effects of hyperoxia and E2 on HCEnCs from female donors suggest cell sex-specific mechanisms of toxicity and hormonal influences.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
Update of
-
Selective effects of estradiol on human corneal endothelial cells.bioRxiv [Preprint]. 2023 Apr 28:2023.04.27.538629. doi: 10.1101/2023.04.27.538629. bioRxiv. 2023. Update in: Sci Rep. 2023 Sep 15;13(1):15279. doi: 10.1038/s41598-023-42290-z. PMID: 37162976 Free PMC article. Updated. Preprint.
Similar articles
-
Selective effects of estradiol on human corneal endothelial cells.bioRxiv [Preprint]. 2023 Apr 28:2023.04.27.538629. doi: 10.1101/2023.04.27.538629. bioRxiv. 2023. Update in: Sci Rep. 2023 Sep 15;13(1):15279. doi: 10.1038/s41598-023-42290-z. PMID: 37162976 Free PMC article. Updated. Preprint.
-
Effect of Physiological Oxygen on Primary Human Corneal Endothelial Cell Cultures.Transl Vis Sci Technol. 2022 Feb 1;11(2):33. doi: 10.1167/tvst.11.2.33. Transl Vis Sci Technol. 2022. PMID: 35191961 Free PMC article.
-
Loss of NQO1 generates genotoxic estrogen-DNA adducts in Fuchs Endothelial Corneal Dystrophy.Free Radic Biol Med. 2020 Feb 1;147:69-79. doi: 10.1016/j.freeradbiomed.2019.12.014. Epub 2019 Dec 17. Free Radic Biol Med. 2020. PMID: 31857234 Free PMC article.
-
The pathophysiology of Fuchs' endothelial dystrophy--a review of molecular and cellular insights.Exp Eye Res. 2015 Jan;130:97-105. doi: 10.1016/j.exer.2014.10.023. Epub 2014 Nov 1. Exp Eye Res. 2015. PMID: 25446318 Review.
-
MicroRNA of Epithelial to Mesenchymal Transition in Fuchs' Endothelial Corneal Dystrophy.Genes (Basel). 2022 Sep 23;13(10):1711. doi: 10.3390/genes13101711. Genes (Basel). 2022. PMID: 36292595 Free PMC article. Review.
Cited by
-
Expression of Hormones' Receptors in Human Corneal Endothelium from Fuchs' Dystrophy: A Possible Gender' Association.J Clin Med. 2024 Jun 27;13(13):3787. doi: 10.3390/jcm13133787. J Clin Med. 2024. PMID: 38999352 Free PMC article.
-
Genetic and Demographic Determinants of Fuchs Endothelial Corneal Dystrophy Risk and Severity.JAMA Ophthalmol. 2025 Apr 1;143(4):338-347. doi: 10.1001/jamaophthalmol.2025.0109. JAMA Ophthalmol. 2025. PMID: 40079965 Free PMC article.
-
Associations between the incidence of Fuchs' endothelial corneal dystrophy and menopausal hormone therapy use and exposure to endogenous estrogen.Maturitas. 2025 Jan;191:108132. doi: 10.1016/j.maturitas.2024.108132. Epub 2024 Oct 15. Maturitas. 2025. PMID: 39522475 Free PMC article.
-
Estrogen hindrance escalates inflammation and neurodegeneration in the hippocampal regions of collagen-induced arthritis female Sprague-Dawley rats.Pflugers Arch. 2025 Feb;477(2):317-332. doi: 10.1007/s00424-024-03032-w. Epub 2024 Nov 21. Pflugers Arch. 2025. PMID: 39570400
-
Associations between measures of oestrogen exposure and severity of Fuchs endothelial corneal dystrophy.BMJ Open Ophthalmol. 2025 May 14;10(1):e001884. doi: 10.1136/bmjophth-2024-001884. BMJ Open Ophthalmol. 2025. PMID: 40374208 Free PMC article.
References
-
- 2019 Eye Banking Statistical Report. (Eye Bank Association of America, Washington, DC, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

