Assessment of spinal cord injury using ultrasound elastography in a rabbit model in vivo
- PMID: 37714920
- PMCID: PMC10504274
- DOI: 10.1038/s41598-023-41172-8
Assessment of spinal cord injury using ultrasound elastography in a rabbit model in vivo
Abstract
The effect of the mechanical micro-environment on spinal cord injury (SCI) and treatment effectiveness remains unclear. Currently, there are limited imaging methods that can directly assess the localized mechanical behavior of spinal cords in vivo. In this study, we apply new ultrasound elastography (USE) techniques to assess SCI in vivo at the site of the injury and at the time of one week post injury, in a rabbit animal model. Eleven rabbits underwent laminectomy procedures. Among them, spinal cords of five rabbits were injured during the procedure. The other six rabbits were used as control. Two neurological statuses were achieved: non-paralysis and paralysis. Ultrasound data were collected one week post-surgery and processed to compute strain ratios. Histologic analysis, mechanical testing, magnetic resonance imaging (MRI), computerized tomography and MRI diffusion tensor imaging (DTI) were performed to validate USE results. Strain ratios computed via USE were found to be significantly different in paralyzed versus non-paralyzed rabbits. The myelomalacia histologic score and spinal cord Young's modulus evaluated in selected animals were in good qualitative agreement with USE assessment. It is feasible to use USE to assess changes in the spinal cord of the presented animal model. In the future, with more experimental data available, USE may provide new quantitative tools for improving SCI diagnosis and prognosis.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
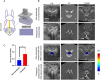
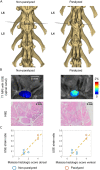
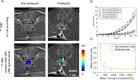

Similar articles
-
The role of diffusion tensor imaging in the diagnosis, prognosis, and assessment of recovery and treatment of spinal cord injury: a systematic review.Neurosurg Focus. 2019 Mar 1;46(3):E7. doi: 10.3171/2019.1.FOCUS18591. Neurosurg Focus. 2019. PMID: 30835681
-
Diffusion tensor imaging as a biomarker for assessing neuronal stem cell treatments affecting areas distal to the site of spinal cord injury.J Neurosurg Spine. 2017 Feb;26(2):243-251. doi: 10.3171/2016.5.SPINE151319. Epub 2016 Sep 30. J Neurosurg Spine. 2017. PMID: 27689421
-
Diffusion tensor imaging of spinal cord parenchyma lesion in rat with chronic spinal cord injury.Magn Reson Imaging. 2018 Apr;47:25-32. doi: 10.1016/j.mri.2017.11.009. Epub 2017 Nov 14. Magn Reson Imaging. 2018. PMID: 29154896
-
The role of spinal cord tractography in detecting lesions following selective bladder afferent and efferent fibers injury: A novel method for induction of neurogenic lower urinary tract dysfunction in rabbit.Neurourol Urodyn. 2022 Sep;41(7):1539-1552. doi: 10.1002/nau.25009. Epub 2022 Jul 17. Neurourol Urodyn. 2022. PMID: 35842827
-
Diffusion Tensor Imaging in Acute Spinal Cord Injury: A Review of Animal and Human Studies.J Neurotrauma. 2019 Aug 1;36(15):2279-2286. doi: 10.1089/neu.2019.6379. Epub 2019 May 6. J Neurotrauma. 2019. PMID: 30950317 Review.
Cited by
-
Neurochemical atlas of the rabbit spinal cord.Brain Struct Funct. 2024 Nov;229(8):2011-2027. doi: 10.1007/s00429-024-02842-z. Epub 2024 Aug 8. Brain Struct Funct. 2024. PMID: 39115602
-
Mathematical Models for Ultrasound Elastography: Recent Advances to Improve Accuracy and Clinical Utility.Bioengineering (Basel). 2024 Sep 30;11(10):991. doi: 10.3390/bioengineering11100991. Bioengineering (Basel). 2024. PMID: 39451367 Free PMC article. Review.
References
-
- Fehlings MG, Vaccaro AR, Boakye M. Essentials of Spinal Cord Injury: Basic Research to Clinical Practice. Georg Thieme Verlag; 2012.
-
- Schwab, M. E. & Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. (1996). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

