The artificial pancreas: two alternative approaches to achieve a fully closed-loop system with optimal glucose control
- PMID: 37715091
- PMCID: PMC10904408
- DOI: 10.1007/s40618-023-02193-2
The artificial pancreas: two alternative approaches to achieve a fully closed-loop system with optimal glucose control
Abstract
Introduction: Diabetes mellitus type 1 is a chronic disease that implies mandatory external insulin delivery. The patients must monitor their blood glucose levels and administer appropriate insulin boluses to keep their blood glucose within the desired range. It requires a lot of time and endeavour, and many patients struggle with suboptimal glucose control despite all their efforts.
Materials and methods: This narrative review combines existing knowledge with new discoveries from animal experiments.
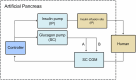
Discussion: In the last decade, artificial pancreas (AP) devices have been developed to improve glucose control and relieve patients of the constant burden of managing their disease. However, a feasible and fully automated AP is yet to be developed. The main challenges preventing the development of a true, subcutaneous (SC) AP system are the slow dynamics of SC glucose sensing and particularly the delay in effect on glucose levels after SC insulin infusions. We have previously published studies on using the intraperitoneal space for an AP; however, we further propose a novel and potentially disruptive way to utilize the vasodilative properties of glucagon in SC AP systems.
Conclusion: This narrative review presents two lesser-explored viable solutions for AP systems and discusses the potential for improvement toward a fully automated system: A) using the intraperitoneal approach for more rapid insulin absorption, and B) besides using glucagon to treat and prevent hypoglycemia, also administering micro-boluses of glucagon to increase the local SC blood flow, thereby accelerating SC insulin absorption and SC glucose sensor site dynamics.
Keywords: Blood glucose; Diabetes mellitus; Glucagon; Insulin infusion system; Type 1.
© 2023. The Author(s).
Conflict of interest statement
The Norwegian University of Science and Technology (NTNU), the university where the several of the author reside, has a patent filed related to the use of micro-boluses of glucagon. The authors SMC, SCC, and ALF are some of the inventors. The remaining authors have no conflicts of interest to declare.
Figures

References
-
- Heise T, Linnebjerg H, Coutant D, et al. Ultra rapid lispro lowers postprandial glucose and more closely matches normal physiological glucose response compared to other rapid insulin analogues: a phase 1 randomized, crossover study. Diabetes Obes Metab. 2020;22(10):1789–1798. doi: 10.1111/dom.14094. - DOI - PMC - PubMed
-
- Løvaas KF, Madsen TV, Tollånes MC, et al. 2021 Norsk diabetesregister for voksne-Årsrapport. Nasjonalt servicemiljø for medisinske kvalitetsregistre. https://www.noklus.no/media/3kcdvzhi/type1_a-rsrapport-norsk-diabetesreg.... Accessed 25 Oct 2022
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

