Uterine WNTS modulates fibronectin binding activity required for blastocyst attachment through the WNT/CA2+ signaling pathway in mice
- PMID: 37715251
- PMCID: PMC10503100
- DOI: 10.1186/s12958-023-01135-0
Uterine WNTS modulates fibronectin binding activity required for blastocyst attachment through the WNT/CA2+ signaling pathway in mice
Abstract
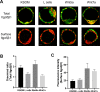
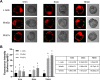
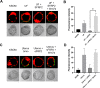
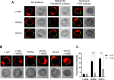
Adhesion of the implanting blastocyst involves the interaction between integrin proteins expressed by trophoblast cells and components present in the basement membrane of the endometrial luminal epithelium. Although several factors regulating integrins and their adhesion to fibronectin are already known, we showed that Wnt signaling is involved in the regulation of blastocyst adhesion through the trafficking of integrins expressed by trophoblast cells. Localization of Itgα5β1 by immunofluorescence and FN-binding assays were conducted on peri-implantation blastocysts treated with either Wnt5a or Wnt7a proteins. Both Wnt5a and Wnt7a induced a translocation of Itgα5β1 at the surface of the blastocyst and an increase in FN-binding activity. We further demonstrated that uterine fluid is capable of inducing integrin translocation and this activity can be specifically inhibited by the Wnt inhibitor sFRP2. To identify the Wnt signaling pathway involved in this activity, blastocysts were incubated with inhibitors of either p38MAPK, PI3K pathway or CamKII prior to the addition of Wnts. Whereas inhibition of p38MAPK and PI3K had not effect, inhibition of CamKII reduced FN-binding activity induced by Wnts. Finally, we demonstrated that inhibition of Wnts by sFRP2 reduced the binding efficiency of the blastocyst to uterine epithelial cells. Our findings provide new insight into the mechanism that regulates integrin trafficking and FN-binding activity and identifies Wnts as a key player in blastocyst attachment to the uterine epithelium.
Keywords: Embryo implantation; Fibronectin binding; Integrins; Mouse blastocyst; Non-canonical wnt signaling; Trophectoderm; Wnt signaling; Wnt/Ca2+.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells.Dev Biol. 2002 May 15;245(2):270-9. doi: 10.1006/dbio.2002.0644. Dev Biol. 2002. PMID: 11977980
-
Trophoblast adhesion of the peri-implantation mouse blastocyst is regulated by integrin signaling that targets phospholipase C.Dev Biol. 2007 Feb 1;302(1):143-53. doi: 10.1016/j.ydbio.2006.09.015. Epub 2006 Sep 14. Dev Biol. 2007. PMID: 17027741 Free PMC article.
-
Insulin-like growth factor 1 increases apical fibronectin in blastocysts to increase blastocyst attachment to endometrial epithelial cells in vitro.Hum Reprod. 2015 Feb;30(2):284-98. doi: 10.1093/humrep/deu309. Epub 2014 Nov 28. Hum Reprod. 2015. PMID: 25432925
-
Integrin-mediated adhesion and signaling during blastocyst implantation.Cells Tissues Organs. 2002;172(3):190-201. doi: 10.1159/000066970. Cells Tissues Organs. 2002. PMID: 12476048 Review.
-
Wnt signaling and cell-matrix adhesion.Curr Mol Med. 2014 Feb;14(2):209-20. doi: 10.2174/1566524014666140128105352. Curr Mol Med. 2014. PMID: 24467207 Review.
Cited by
-
Wnt7a can upregulate cell adhesion and migration related genes expression and facilitate the repair of corneal epithelial cells after injury.Int Ophthalmol. 2025 Apr 7;45(1):149. doi: 10.1007/s10792-025-03506-5. Int Ophthalmol. 2025. PMID: 40192882
-
Extracellular matrix and pregnancy: functions and opportunities caught in the net.Reprod Biol Endocrinol. 2025 Feb 14;23(1):24. doi: 10.1186/s12958-025-01348-5. Reprod Biol Endocrinol. 2025. PMID: 39953593 Free PMC article. Review.
-
WD-repeat containing protein-61 regulates endometrial epithelial cell adhesion indicating an important role in receptivity.Mol Hum Reprod. 2024 Nov 14;30(11):gaae039. doi: 10.1093/molehr/gaae039. Mol Hum Reprod. 2024. PMID: 39531333 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

