Bald sea urchin disease shifts the surface microbiome on purple sea urchins in an aquarium
- PMID: 37715299
- PMCID: PMC10550250
- DOI: 10.1093/femspd/ftad025
Bald sea urchin disease shifts the surface microbiome on purple sea urchins in an aquarium
Abstract
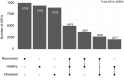
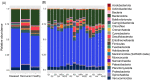
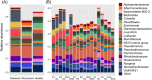
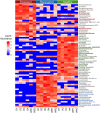
Bald sea urchin disease (BSUD) is most likely a bacterial infection that occurs in a wide range of sea urchin species and causes the loss of surface appendages. The disease has a variety of additional symptoms, which may be the result of the many bacteria that are associated with BSUD. Previous studies have investigated causative agents of BSUD, however, there are few reports on the surface microbiome associated with the infection. Here, we report changes to the surface microbiome on purple sea urchins in a closed marine aquarium that contracted and then recovered from BSUD in addition to the microbiome of healthy sea urchins in a separate aquarium. 16S rRNA gene sequencing shows that microhabitats of different aquaria are characterized by different microbial compositions, and that diseased, recovered, and healthy sea urchins have distinct microbial compositions, which indicates that there is a correlation between microbial shifts and recovery from disease.
Keywords: 16S rRNA high throughput sequencing; Strongylocentrotus purpuratus; bacterial infection; echinoderm; microbiome.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Spotting disease disrupts the microbiome of infected purple sea urchins, Strongylocentrotus purpuratus.BMC Microbiol. 2024 Jan 4;24(1):11. doi: 10.1186/s12866-023-03161-9. BMC Microbiol. 2024. PMID: 38172649 Free PMC article.
-
Microbial Composition and Genes for Key Metabolic Attributes in the Gut Digesta of Sea Urchins Lytechinus variegatus and Strongylocentrotus purpuratus Using Shotgun Metagenomics.Curr Issues Mol Biol. 2021 Aug 26;43(2):978-995. doi: 10.3390/cimb43020070. Curr Issues Mol Biol. 2021. PMID: 34563039 Free PMC article.
-
High-Throughput Sequencing Analysis Revealed a Preference for Animal-Based Food in Purple Sea Urchins.Biology (Basel). 2024 Aug 15;13(8):623. doi: 10.3390/biology13080623. Biology (Basel). 2024. PMID: 39194561 Free PMC article.
-
Ocean acidification research in the 'post-genomic' era: Roadmaps from the purple sea urchin Strongylocentrotus purpuratus.Comp Biochem Physiol A Mol Integr Physiol. 2015 Jul;185:33-42. doi: 10.1016/j.cbpa.2015.03.007. Epub 2015 Mar 13. Comp Biochem Physiol A Mol Integr Physiol. 2015. PMID: 25773301 Review.
-
Sea Urchin as a Universal Model for Studies of Gene Networks.Front Genet. 2021 Jan 20;11:627259. doi: 10.3389/fgene.2020.627259. eCollection 2020. Front Genet. 2021. PMID: 33552139 Free PMC article. Review.
Cited by
-
Recombinant SpTransformer proteins are functionally diverse for binding and phagocytosis by three subtypes of sea urchin phagocytes.Front Immunol. 2024 Apr 29;15:1372904. doi: 10.3389/fimmu.2024.1372904. eCollection 2024. Front Immunol. 2024. PMID: 38742116 Free PMC article.
-
Investigating the influence of Diadematidae scuticociliatosis on host microbiome composition.mSystems. 2025 Mar 18;10(3):e0141824. doi: 10.1128/msystems.01418-24. Epub 2025 Feb 19. mSystems. 2025. PMID: 39969199 Free PMC article.
-
Spotting disease disrupts the microbiome of infected purple sea urchins, Strongylocentrotus purpuratus.BMC Microbiol. 2024 Jan 4;24(1):11. doi: 10.1186/s12866-023-03161-9. BMC Microbiol. 2024. PMID: 38172649 Free PMC article.
References
-
- Azzolina JF, Boudouresque CHF, Nedelec H. Dynamique des populations de Paracentrotus lividus dans la baie de Port-Cros (Var): données préliminaires. Sci Rep Port-Cros Natl Park. 1985;11:61–81.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

