METTL3-mediated N6-methyladenosine exacerbates ferroptosis via m6A-IGF2BP2-dependent mitochondrial metabolic reprogramming in sepsis-induced acute lung injury
- PMID: 37715457
- PMCID: PMC10504453
- DOI: 10.1002/ctm2.1389
METTL3-mediated N6-methyladenosine exacerbates ferroptosis via m6A-IGF2BP2-dependent mitochondrial metabolic reprogramming in sepsis-induced acute lung injury
Abstract
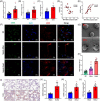
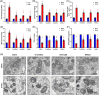
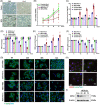
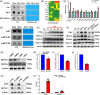
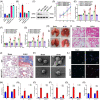
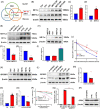
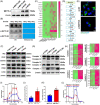
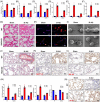
Neutrophil extracellular traps (NETs), released by polymorphonuclear neutrophils (PMNs), exert a robust antimicrobial function in infectious diseases such as sepsis. NETs also contribute to the pathogenesis and exacerbation of sepsis. Although the lung is highly vulnerable to infections, few studies have explored the role of NETs in sepsis-induced acute lung injury (SI-ALI). We demonstrate that NETs induce SI-ALI via enhanced ferroptosis in alveolar epithelial cells. Our findings reveal that the excessive release of NETs in patients and mice with SI-ALI is accompanied by upregulation of ferroptosis depending on METTL3-induced m6A modification of hypoxia-inducible factor-1α (HIF-1α) and subsequent mitochondrial metabolic reprogramming. In addition to conducting METTL3 overexpression and knockdown experiments in vitro, we also investigated the impact of ferroptosis on SI-ALI caused by NETs in a caecum ligation and puncture (CLP)-induced SI-ALI model using METTL3 condition knockout (CKO) mice and wild-type mice. Our results indicate the crucial role of NETs in the progression of SI-ALI via NET-activated METTL3 m6A-IGF2BP2-dependent m6A modification of HIF-1α, which further contributes to metabolic reprogramming and ferroptosis in alveolar epithelial cells.
Keywords: N6-methylation; ferroptosis; metabolic reprogramming; neutrophil extracellular traps; sepsis-induced acute lung injury.
© 2023 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures
Similar articles
-
Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury.Redox Biol. 2024 Aug;74:103194. doi: 10.1016/j.redox.2024.103194. Epub 2024 May 16. Redox Biol. 2024. PMID: 38852200 Free PMC article.
-
Neutrophil extracellular traps mediate m6A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells.Int J Biol Sci. 2022 May 9;18(8):3337-3357. doi: 10.7150/ijbs.69141. eCollection 2022. Int J Biol Sci. 2022. PMID: 35637949 Free PMC article.
-
METTL4 mediated-N6-methyladenosine promotes acute lung injury by activating ferroptosis in alveolar epithelial cells.Free Radic Biol Med. 2024 Mar;213:90-101. doi: 10.1016/j.freeradbiomed.2024.01.013. Epub 2024 Jan 14. Free Radic Biol Med. 2024. PMID: 38224757
-
The role of neutrophil extracellular traps in acute lung injury.Front Immunol. 2022 Jul 29;13:953195. doi: 10.3389/fimmu.2022.953195. eCollection 2022. Front Immunol. 2022. PMID: 35967320 Free PMC article. Review.
-
Role and mechanisms of autophagy, ferroptosis, and pyroptosis in sepsis-induced acute lung injury.Front Pharmacol. 2024 Aug 5;15:1415145. doi: 10.3389/fphar.2024.1415145. eCollection 2024. Front Pharmacol. 2024. PMID: 39161900 Free PMC article. Review.
Cited by
-
Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury.Redox Biol. 2024 Aug;74:103194. doi: 10.1016/j.redox.2024.103194. Epub 2024 May 16. Redox Biol. 2024. PMID: 38852200 Free PMC article.
-
Advances in neutrophil extracellular traps and ferroptosis in sepsis-induced cardiomyopathy.Front Immunol. 2025 Apr 28;16:1590313. doi: 10.3389/fimmu.2025.1590313. eCollection 2025. Front Immunol. 2025. PMID: 40356926 Free PMC article. Review.
-
Adipose-derived mesenchymal stem cell-derived extracellular vesicles carry microRNA-214-3p to target GSTZ1 to curb ferroptosis in lung epithelial cells during sepsis.Cytotechnology. 2025 Aug;77(4):132. doi: 10.1007/s10616-025-00793-9. Epub 2025 Jul 1. Cytotechnology. 2025. PMID: 40611882
-
Ferritin-mediated neutrophil extracellular traps formation and cytokine storm via macrophage scavenger receptor in sepsis-associated lung injury.Cell Commun Signal. 2024 Feb 2;22(1):97. doi: 10.1186/s12964-023-01440-6. Cell Commun Signal. 2024. PMID: 38308264 Free PMC article.
-
METTL16/IGF2BP2 axis enhances malignant progression and DDP resistance through up-regulating COL4A1 by mediating the m6A methylation modification of LAMA4 in hepatocellular carcinoma.Cell Div. 2025 Apr 18;20(1):9. doi: 10.1186/s13008-025-00152-2. Cell Div. 2025. PMID: 40251670 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
