NLGN4X TCR transgenic T cells to treat gliomas
- PMID: 37715782
- PMCID: PMC10836769
- DOI: 10.1093/neuonc/noad172
NLGN4X TCR transgenic T cells to treat gliomas
Abstract
Background: Neuroligin 4 X-linked (NLGN4X) harbors a human leukocyte antigen (HLA)-A*02-restricted tumor-associated antigen, overexpressed in human gliomas, that was found to induce specific cytotoxic T cell responses following multi-peptide vaccination in patients with newly diagnosed glioblastoma.
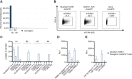
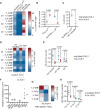
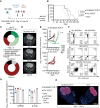
Methods: T cell receptor (TCR) discovery was performed using droplet-based single-cell TCR sequencing of NLGN4X-tetramer-sorted T cells postvaccination. The identified TCR was delivered to Jurkat T cells and primary human T cells (NLGN4X-TCR-T). Functional profiling of NLGN4X-TCR-T was performed by flow cytometry and cytotoxicity assays. Therapeutic efficacy of intracerebroventricular NLGN4X-TCR-T was assessed in NOD scid gamma (NSG) major histocompatibility complex (MHC) I/II knockout (KO) (NSG MHC I/II KO) mice bearing NLGN4X-expressing experimental gliomas.
Results: An HLA-A*02-restricted vaccine-induced T cell receptor specifically binding NLGN4X131-139 was applied for preclinical therapeutic use. Reactivity, cytotoxicity, and polyfunctionality of this NLGN4X-specific TCR are demonstrated in various cellular models. Intracerebroventricular administration of NLGN4X-TCR-T prolongs survival and leads to an objective response rate of 44.4% in experimental glioma-bearing NSG MHC I/II KO mice compared to 0.0% in control groups.
Conclusion: NLGN4X-TCR-T demonstrate efficacy in a preclinical glioblastoma model. On a global scale, we provide the first evidence for the therapeutic retrieval of vaccine-induced human TCRs for the off-the-shelf treatment of glioblastoma patients.Keywords cell therapy | glioblastoma | T cell receptor | tumor antigen.
Keywords: Keywords cell therapy; T cell receptor; glioblastoma; tumor antigen.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
T-cell receptor-derived binding polypeptides PCT/EP2023/061716 to L.B., E.G., M.P, Y.C.C, W.W., M.K., and C.K. Non-integrating DNA vectors for the genetic modification of cells WO2019060253A1 to R.H. Honoraria from Adaptive Biotechnologies to L.B. Consulting fees from Servier, Bayer, GSK, MSD, and Roche to W.W. Royalties are received from The Jackson Laboratory from their licensing NSG MHC class I/II knockout mice to For Profit Companies by L.D.S.
Figures



References
-
- Hilf N, Kuttruff-Coqui S, Frenzel K, et al. . Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. Jan 2019;565(7738):240–245. - PubMed
-
- Vitanza NA, Johnson AJ, Wilson AL, et al. . Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med. 2021;27(9):1544–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

