Assessing suffering of patients on cancer treatment and of those no longer treated using ESAS-Total Care (TC)
- PMID: 37715838
- PMCID: PMC10505109
- DOI: 10.1007/s00520-023-08035-4
Assessing suffering of patients on cancer treatment and of those no longer treated using ESAS-Total Care (TC)
Abstract
Aim: The aim of the study was to assess the suffering of patients on oncologic treatment and of those no longer on treatment. Preliminarily, we aimed to confirm the psychometric properties of Edmonton Symptom Assessment System-Total Care (ESAS-TC) in different stages of the disease. The ESAS-TC screens physical and psychological symptoms, but also spiritual pain, discomfort deriving from financial problems associated with illness, and suffering related to social isolation.
Methods: A sample of consecutive advanced cancer patients on oncologic therapies treated at the Internistic and Geriatric Supportive Care Unit (IGSCU) of Istituto Nazionale dei Tumori, Milano, and of terminal patients no longer on treatment and cared for by the Fondazione ANT palliative home care team were asked to fill the ESAS-TC. In order to strengthen the previous validation study of the ESAS-TC, 3-ULS (to assess social isolation), JSWBS (to assess spiritual well-being), COST-IT (to assess financial distress), and KPS (to assess functional status) were administered too.
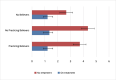
Results: The questionnaires were self-reported by 108 patients on treatment (52% >60 years old, female 53%, and 61% with KPS 90-100) and by 94 home care patients (71% >60 years old, female 51%, and 68% with KPS 10-50). The sound psychometric characteristics of ESAS-TC were confirmed. Patients on treatment showed lower total ESAS-TC score (19.3 vs 52.7, p<.001) after controlling for age and functional status, and lower financial distress (p.<001). Financial distress, spiritual suffering, and social isolation, after controlling for age, showed a significantly higher score in home care patients.
Conclusions: Only through an adequate routine assessment with validated tools is it possible to detect total suffering, the "Total pain" of patients, and treat it through a multidisciplinary approach. The study confirms the reliability and validity of the Italian version of ESAS-TC and the importance of supportive and early palliative care fully integrated with oncological treatment.
Keywords: Financial; Home palliative care; Isolation; Oncologic treatments; Psychometric validation; Spirituality; Terminal cancer; Total care.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

