Parkinson's disease-linked parkin mutation disrupts recycling of synaptic vesicles in human dopaminergic neurons
- PMID: 37716354
- PMCID: PMC11977536
- DOI: 10.1016/j.neuron.2023.08.018
Parkinson's disease-linked parkin mutation disrupts recycling of synaptic vesicles in human dopaminergic neurons
Abstract
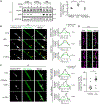
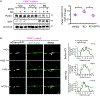
Parkin-mediated mitophagy has been studied extensively, but whether mutations in parkin contribute to Parkinson's disease pathogenesis through alternative mechanisms remains unexplored. Using patient-derived dopaminergic neurons, we found that phosphorylation of parkin by Ca2+/calmodulin-dependent protein kinase 2 (CaMK2) at Ser9 leads to activation of parkin in a neuronal-activity-dependent manner. Activated parkin ubiquitinates synaptojanin-1, facilitating its interaction with endophilin A1 and synaptic vesicle recycling. Neurons from PD patients with mutant parkin displayed defective recycling of synaptic vesicles, leading to accumulation of toxic oxidized dopamine that was attenuated by boosting endophilin A1 expression. Notably, combined heterozygous parkin and homozygous PTEN-induced kinase 1 (PINK1) mutations led to earlier disease onset compared with homozygous mutant PINK1 alone, further underscoring a PINK1-independent role for parkin in contributing to disease. Thus, this study identifies a pathway for selective activation of parkin at human dopaminergic synapses and highlights the importance of this mechanism in the pathogenesis of Parkinson's disease.
Keywords: CaMK2-mediated activation of parkin; PINK1-independent; Parkinson’s disease; human dopaminergic neurons; synaptic dysfunction; toxic oxidized dopamine.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.K. is the founder and scientific advisory board chair of Vanqua Bio, serves on the scientific advisory boards of The Silverstein Foundation, Intellia Therapeutics, AcureX, and is a venture partner at OrbiMed.
Figures





References
-
- Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, et al. (2012). A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One 7, e36458. 10.1371/journal.pone.0036458. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

