Effects of Bifidobacterium BL21 and Lacticaseibacillus LRa05 on gut microbiota in type 2 diabetes mellitus mice
- PMID: 37716924
- PMCID: PMC10505128
- DOI: 10.1186/s13568-023-01603-1
Effects of Bifidobacterium BL21 and Lacticaseibacillus LRa05 on gut microbiota in type 2 diabetes mellitus mice
Abstract
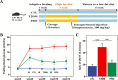
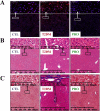
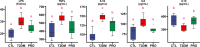
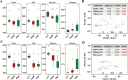
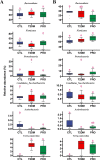
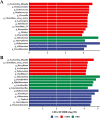
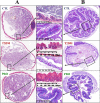
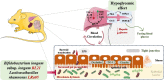
Gut dysbiosis causes damage to the intestinal barrier and is associated with type 2 diabetes mellitus (T2DM). We tested the potential protective effects of probiotic BL21 and LRa05 on gut microbiota in type 2 diabetes mellitus mice and determined whether these effects were related to the modulation of gut microbiota.Thirty specific pathogen-free C57BL/6J mice were randomly allocated to three groups-the (CTL) control group, HFD/STZ model (T2DM) group, and HFD/STZ-probiotic intervention (PRO) group-and intragastrically administered strains BL21 and LRa05 for 11 weeks. The administration of strains BL21 and LRa05 significantly regulated blood glucose levels, accompanied by ameliorated oxidative stress in mice. The BL21/LRa05-treated mice were protected from liver, cecal, and colon damage. Microbiota analysis showed that the cecal and fecal microbiota of the mice presented significantly different spatial distributions from one another. Principal coordinate analysis results indicated that both T2DM and the BL21/LRa05 intervention had significant effects on the cecal contents and fecal microbiota structure. In terms of the fecal microbiota, an abundance of Akkermansia and Anaeroplasma was noted in the PRO group. In terms of the cecal content microbiota, enrichment of Akkermansia, Desulfovibrio, Bifidobacterium, Lactobacillus, and Limosilactobacillus was noted in the PRO group. The probiotics BL21 and LRa05 prevent or ameliorate T2DM by regulating the intestinal flora and reducing inflammation and oxidative stress. Our results suggest that BL21 and LRa05 colonize in the cecum. Thus, BL21/LRa05 combined with probiotics having a strong ability to colonize in the colon may achieve better therapeutic effects in T2DM. Our study illustrated the feasibility and benefits of the combined use of probiotics and implied the importance of intervening at multiple intestinal sites in T2DM mice.
Keywords: Gut microbiota; Inflammation; Probiotics; Spatial structure; T2DM.
© 2023. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
All authors declare that they have no competing interests in the subject matter or materials discussed in this manuscript.
Figures
Similar articles
-
Lacticaseibacillus rhamnosus LRa05 alleviated liver injury in mice with alcoholic fatty liver disease by improving intestinal permeability and balancing gut microbiota.Benef Microbes. 2024 Jul 3;15(5):481-493. doi: 10.1163/18762891-bja00022. Benef Microbes. 2024. PMID: 38960385
-
Lacticaseibacillus rhamnosus LRa05 mediates dynamic regulation of intestinal microbiota in mice with low-dose DSS-induced chronic mild inflammation.Front Microbiol. 2024 Oct 8;15:1483104. doi: 10.3389/fmicb.2024.1483104. eCollection 2024. Front Microbiol. 2024. PMID: 39444683 Free PMC article.
-
Lactobacillus rhamnosus LRa05 Ameliorate Hyperglycemia through a Regulating Glucagon-Mediated Signaling Pathway and Gut Microbiota in Type 2 Diabetic Mice.J Agric Food Chem. 2021 Aug 11;69(31):8797-8806. doi: 10.1021/acs.jafc.1c02925. Epub 2021 Aug 2. J Agric Food Chem. 2021. PMID: 34340304
-
Gut microbiota and probiotics: Focus on diabetes mellitus.Crit Rev Food Sci Nutr. 2017 Jul 24;57(11):2296-2309. doi: 10.1080/10408398.2014.934438. Crit Rev Food Sci Nutr. 2017. PMID: 26499995 Review.
-
Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus.Front Cell Infect Microbiol. 2022 Feb 15;12:834485. doi: 10.3389/fcimb.2022.834485. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35242721 Free PMC article. Review.
Cited by
-
Reduction in Serum Concentrations of Uremic Toxins Driven by Bifidobacterium Longum Subsp. Longum BL21 is Associated with Gut Microbiota Changes in a Rat Model of Chronic Kidney Disease.Probiotics Antimicrob Proteins. 2024 Jun 3. doi: 10.1007/s12602-024-10293-5. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38829564
-
Modulatory impact of Bifidobacterium longum subsp. longum BL21 on the gut-brain-ovary axis in polycystic ovary syndrome: insights into metabolic regulation, inflammation mitigation, and neuroprotection.mSphere. 2025 Feb 25;10(2):e0088724. doi: 10.1128/msphere.00887-24. Epub 2025 Feb 3. mSphere. 2025. PMID: 39898662 Free PMC article.
-
Lacticaseibacillus paracasei LC86 mitigates age-related muscle wasting and cognitive impairment in SAMP8 mice through gut microbiota modulation and the regulation of serum inflammatory factors.Front Nutr. 2024 May 30;11:1390433. doi: 10.3389/fnut.2024.1390433. eCollection 2024. Front Nutr. 2024. PMID: 38873561 Free PMC article.
-
Managing Type 2 Diabetes Mellitus via the Regulation of Gut Microbiota: A Chinese Medicine Perspective.Nutrients. 2024 Nov 18;16(22):3935. doi: 10.3390/nu16223935. Nutrients. 2024. PMID: 39599721 Free PMC article. Review.
-
Beneficial Effects of Probiotic Lactobacillus paraplantarum BGCG11 on Pancreatic and Duodenum Function in Diabetic Rats.Int J Mol Sci. 2024 Jul 13;25(14):7697. doi: 10.3390/ijms25147697. Int J Mol Sci. 2024. PMID: 39062940 Free PMC article.
References
-
- Dashtbanei S, Keshtmand Z. A Mixture of Multi-Strain Probiotics (Lactobacillus Rhamnosus, Lactobacillus Helveticus, and Lactobacillus Casei) had anti-inflammatory, anti-apoptotic, and anti-oxidative effects in oxidative injuries induced by cadmium in small intestine and lung. Probiotics Antimicrob Proteins. 2022;15:226–238. doi: 10.1007/s12602-022-09946-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

